General
Control of brain development
- Retinoic acid (vitamin A) is important in controlling the expression of Hox genes, important in the regional control of brain development.
- Induction refers to the influence one embryonic tissue has on another.
- Neural induction denotes the influence of non-neural tissue (e.g. the notochord) on the neural tube.
- Recognition molecules, adhesion molecules, cytoskeletal components, and physical barriers (such as the dorsal and ventral median septa) all play a role on the regulation of neuronal migration.
1st Stage: Dorsal Induction
- 18 to 26 days (3–4 weeks) of gestation.
- Goal to form Neural tube
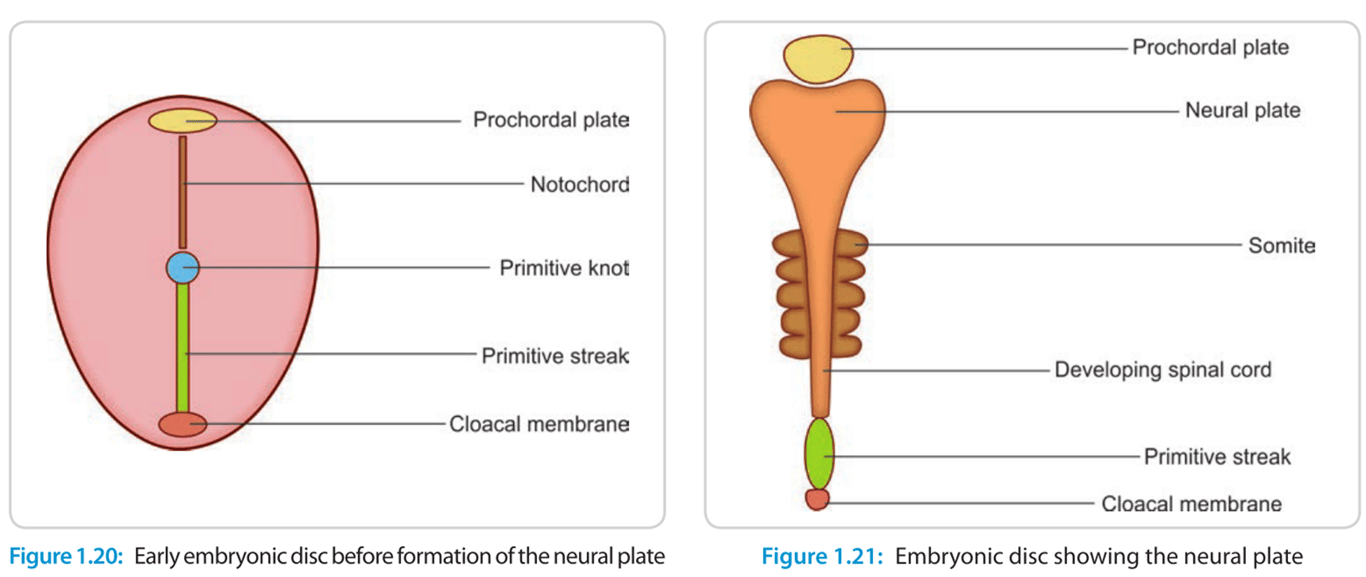
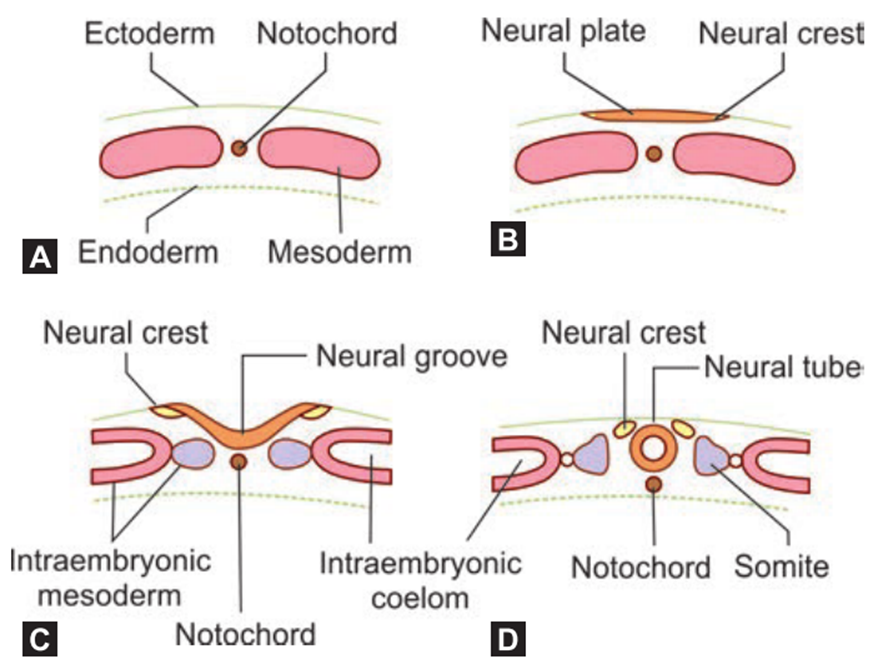
- Mesoderm cells migrate rostrally towards the primitive node (formed during gastrulation), forming the notochord, and also inducing ectoderm to form neuroectoderm comprising the neural plate.
- Neural plate differentiates into two layers which ultimately form the
- Neural tube
- Formation of the neural tube = neurulation
- Involves the neural plate rolling up and fusing.
- Neural groove
- Formed when neural plate becomes depressed along the midline
- Neural fold
- When neural groove deepens and at wk 3 two raised edge raises to form the neural tube
- Divided into
- Formation of the neural tube from the rostral end of the notochord to its caudal end at the L1–L2 level
- Tube is short but gradually gains in length as the embryo grows
- Closure of neural plates to form neural tube
- Neural plate gives rise to both the primary and the secondary neural tubes
- Starts at mid region of the neural plate and advances cranially and caudally in a zipper like fashion
- Neural tube walls thickening: forms brain and the spinal cord
- Neuroepithelial cells that form the walls give rise to
- Neurons
- Macroglia (i.e., astrocytes, oligodendrocytes, and ependymal cells)
- Except:
- Microglia:
- Derived from cells of mesodermal origin that enter the central nervous system (CNS) from the vasculature during development
- Lumen of the tube: forms ventricular system and the central canal.
- Upfolding of the lateral borders of the neural plate
- Neural crest cells
- neural crest: specialized cells of the ectoderm that rises to from the neural plate to form the neural tube
- To achieve this the neural crest cell become free (by losing the property of cell-to-cell adhesiveness)
- They can migrate to distant places throughout the body to form other cells
- Forms most of the peripheral nervous system:
- Sensory ganglion cells of the cranial and spinal nerves
- dorsal root ganglion cells
- Autonomic
- Ganglia
- Post sympathetic neurons
- Adrenal medulla
- Schwann cells
- Melanocytes
- Odontoblast
- Pia mater
- Arachnoid mater
- Pathology
- Hirschsprung’s disease: a disease of neural crest
- Aganglionic megacolon
- Aorticopulmonary septal defects of heart
- Cleft lip
- Cleft palate
- Frontonasal dysplasia
- Neurofibromatosis
- Tumour of adrenal medulla
- Albinism
- Waardenburg syndrome
- Stain for S100 is specific for neural crest cells
- Anterior and posterior neuropores remains in communication with amniotic cavity.
- Closure of the posterior neuropore marks the completion of the first phase (primary neurulation) of neural tube formation
- Anterior neuropore closes: POD 24
- Posterior neuropore closes: POD 26
- Now the foetal spine is covered by ectoderm but the lumbar, sacral, and coccygeal segments have not yet developed.
- Site of the final closure of the caudal neural pore = S2 vertebral level.
- Disjunction:
- Complete separation of the cutaneous ectoderm from the neural ectoderm and closure of the neural tube
- Occurs simultaneously as primary neurulation
- Failure
- Non disjunction: Failure to complete the process of disjunction
- Premature disjunction: failure to completion of disjunction prior to neural tube closure
- Disruption
- Result in a miscarriage.
- Faulty formation of neural tube
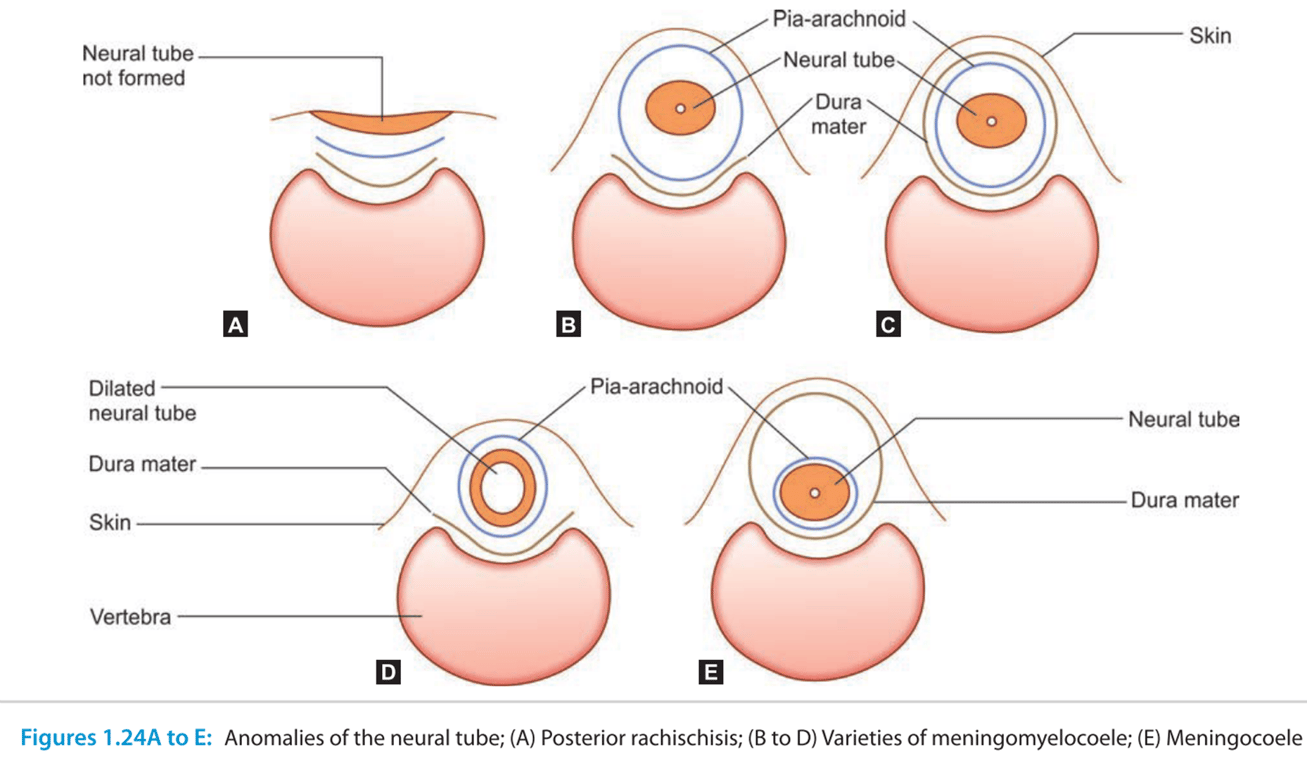
- Posterior rachischisis:
- The whole length of the neural tube remains unclosed.
- Anencephaly:
- Neural tube remains open in the region of the brain because of nonclosure of the anterior neuropore.
- Brain tissue, which is exposed, degenerates.
- Nonclosure of the
- Cranium: Cranium Bifidum → when Neural tissue is exposed → Encephalocele or meningoencephalocele
- Vertebral canal: spinal Bifida → when neural tissue exposed → myelocoele or meningomyelocoele
- Secondary neurulation: Video (start POD 26; end POD ?)
- process in which mesenchymal cells undergo epithelialization and tubulogenesis
- Formation of the
- Bone
- Lower lumbar
- Sacral
- Coccygeal vertebral segments
- Nerve
- Distal cord
- Conus
- Cauda equina and filum
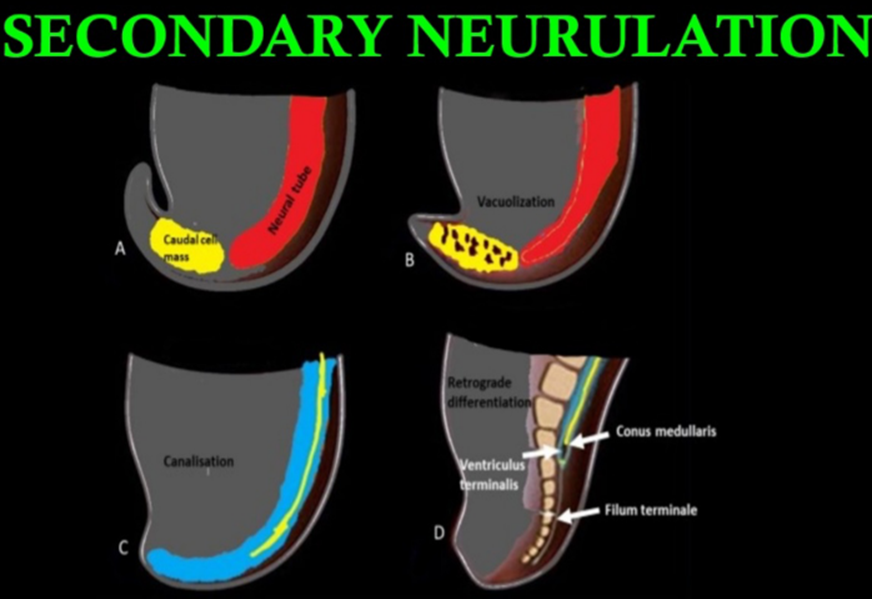
- Formed by an infolding of the neural plate, creating the medullary cord (caudal cell mass), which then undergoes cavitation.
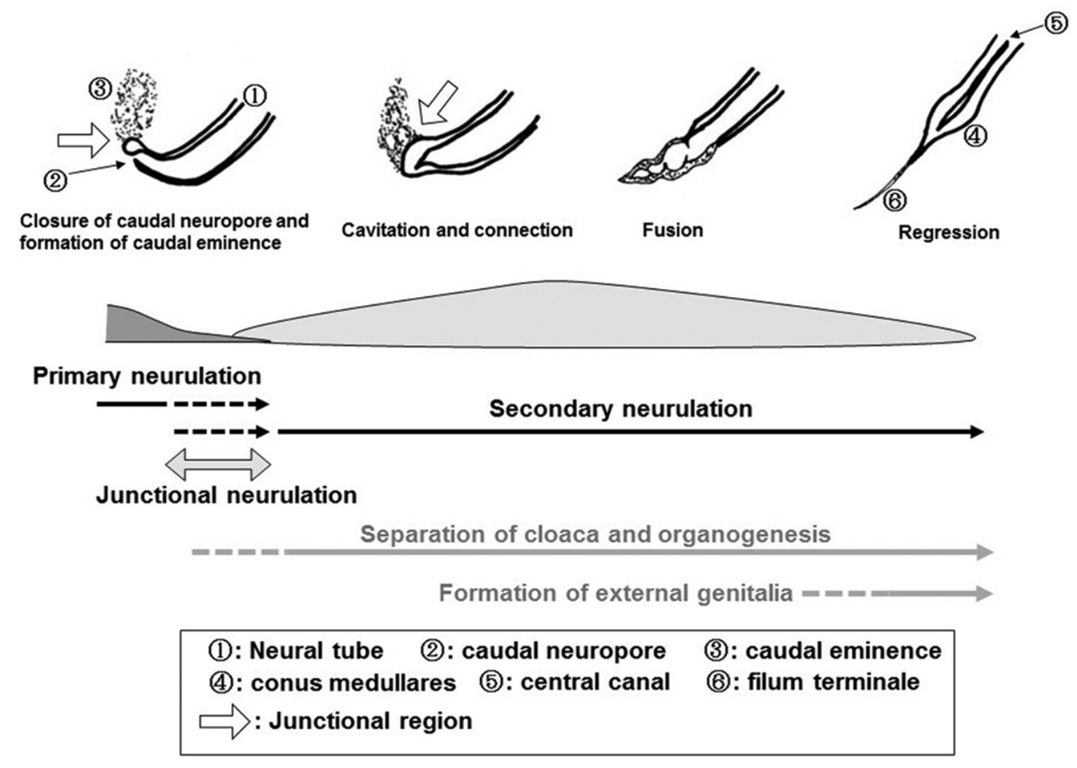
- 4 Phases in the formation of the caudal neural tube (aka secondary neural tube)
- Condensation (POD 20-22)
- Formation of caudal cell mass: A process where the caudal end of the neural tube and the notochord combine into a large aggregate of undifferentiated cells
- This caudal cell mass extends to the level of the tail fold and represents a solid neural cord containing neurons, neural crest, glial cells, and ependymal cells.
- Canalization and connection to central canal (POD 26)
- A process where a series of small vacuoles within the caudal cell mass begin to coalesce and enlarge to achieve an ependymal-lined tube in continuity with the rostral central canal of the previously formed neural tube.
- Leads to the formation of the ventriculus terminalis.
- Fusion of the hollow secondary neural tube with the primary neural tube's neural pore
- Caudal cell mass fuses with primary neural tube in a process called junctional neurulation (NOT the same as the disjunction in primary neurulation)
- Regression (Start POD 28-32; ends at POD 48 -52)
- A process of regression of the structures previously derived during canalization.
- During retrogressive differentiation, there is disappearance of the embryologic tail, whereas the filum terminale, coccygeal ligament, and ventriculus terminalis of the conus remain.
- Because cell rests within the caudal cell mass have totipotent characteristics, a small clone of lipomatous cells developing in the region would not necessarily inhibit the growth of surrounding structures.
- Think of spinal lipomas
- The bony vertebrae develop subsequently, and the sacrococcygeal segments also undergo regressive changes to decrease the number of segments originally present.
- As a result, vertebral malformations are found in conjunction with the neural defects arising during this period.
- Regression of the caudal neural tube forms the filum terminale
- If this fails a low lying conus is formed
Primary neurulation (neural tube formation):
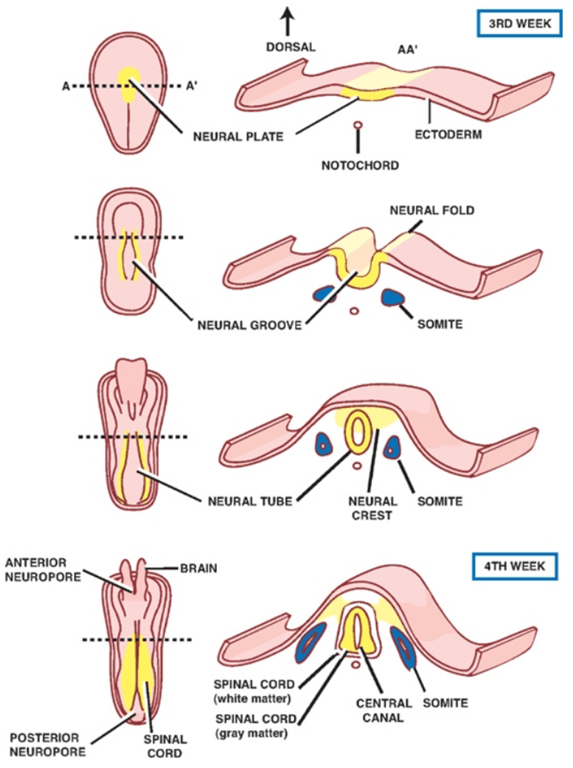
Secondary neurulation
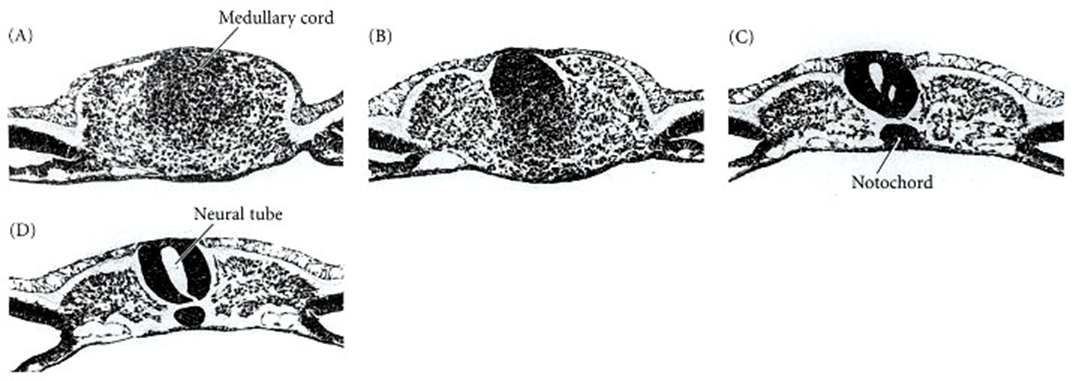
(B) The medullary cord at a slightly more anterior position in the tailbud.
(C) The neural tube is cavitating and the notochord forming.
(D) The lumens coalesce to form the central canal of the neural tube.
- On day 26
- the neural tube closes and this marks the end of dorsal induction.
2nd stage: Ventral Induction
- 4 to 10 weeks of gestation.
- 2 key processes:
- Primary vesicle formation
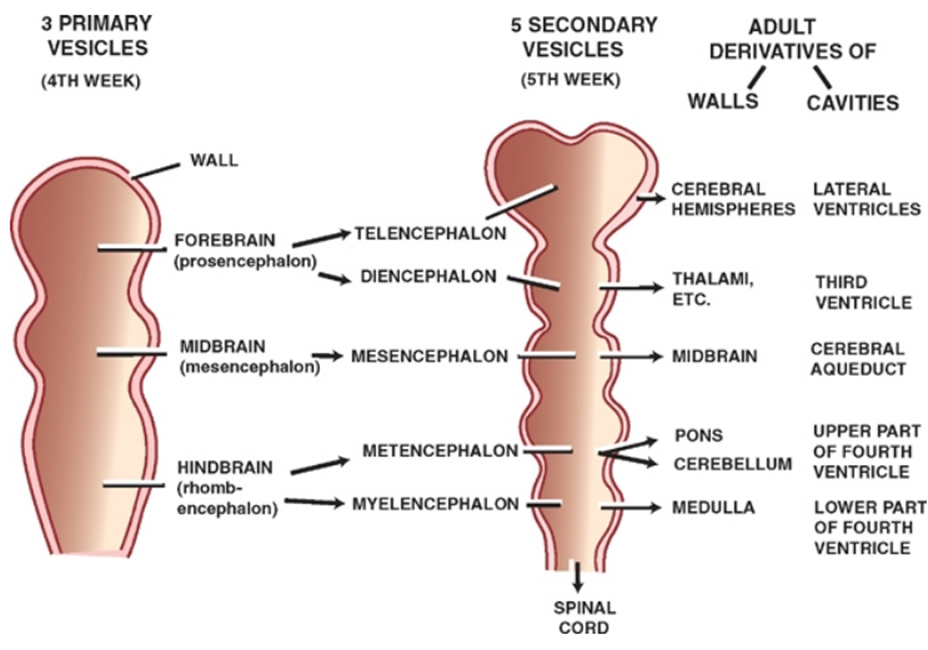
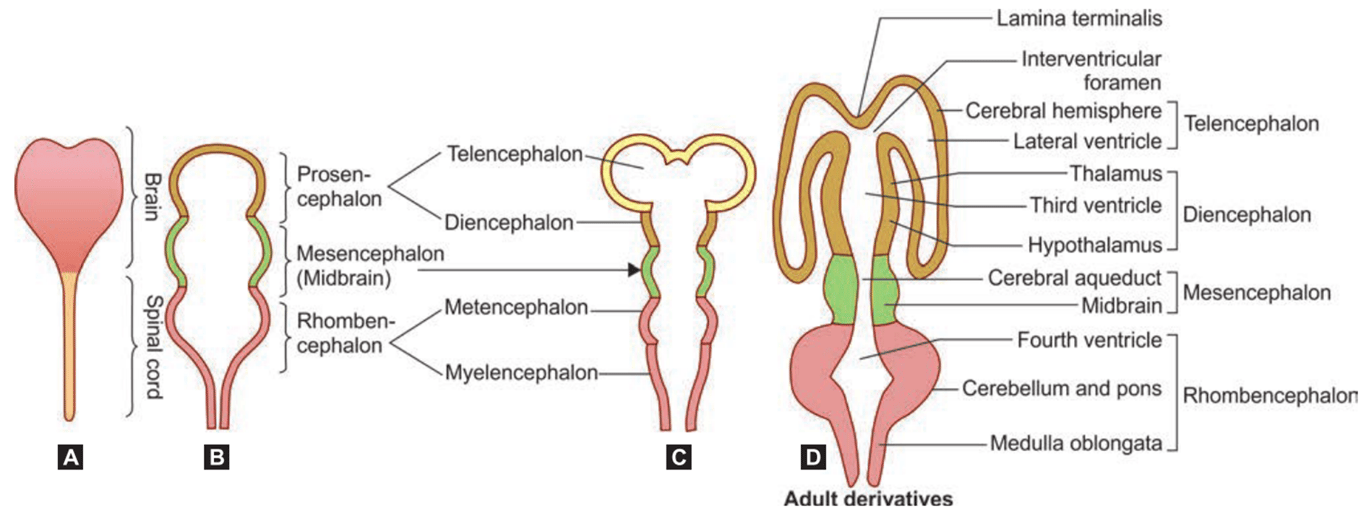
- 4th week:
- 3 primary brain vesicles:
- Forebrain or prosencephalon
- Midbrain or mesencephalon
- Hindbrain or rhombencephalon
- Secondary vesicle formation
- 5th week
- 5 secondary brain vesicle
- Forebrain divides into the
- telencephalon and diencephalon
- Midbrain
- mesencephalon
- Hindbrain divides into the
- Metencephalon
- Myelencephalon
- Cephalic flexure separates the forebrain vs midbrain
- Cervical flexure separates the hindbrain vs spinal cord
- Mesencephalic flexure separates mesencephalon vs rhombencephalon
- Pontine flexure separates the Metencephalon vs Myelencephalon
Vesicles formation
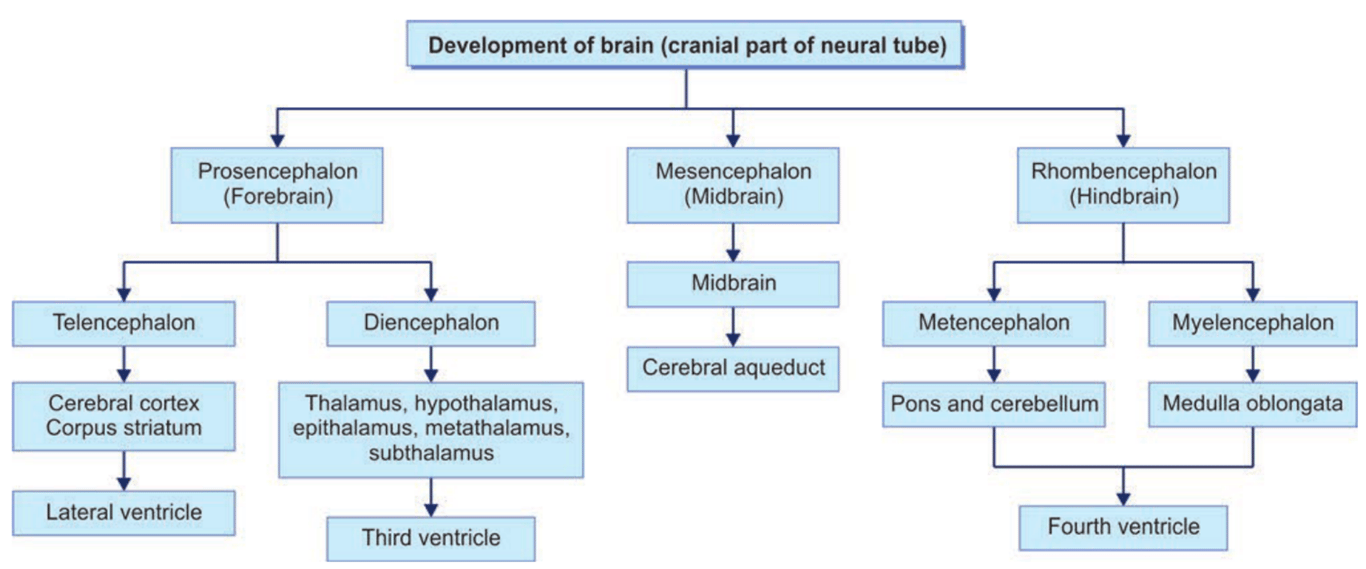
Primary Vesicles | Secondary Vesicles | Adult Derivatives Walls | Adult Derivatives Cavities |
Forebrain (prosencephalon) | Telencephalon Diencephalon | Cerebral hemispheres Thalamus Epithalamus Hypothalamus | Lateral Third |
Midbrain (mesencephalon) | Mesencephalon | Midbrain | Cerebral aqueduct |
Hindbrain (rhombencephalon) | Metencephalon Myelencephalon | Pons Cerebellum Medulla | Fourth Fourth |
Formation of flexures which will divide the brain into different parts
- Approximately half of the end of the neural tube ultimately forms the spinal cord
- Disruption
- Miscarriage typically results
- If the pregnancy continued, the disruption might end in the brain making abnormal cleavages or cysts separating the cerebral hemispheres.
- These events would result in profound impairments.
3rd stage Proliferation
- Initially a single layer of neuroepithelial cells lines the neural tube, bounded by the internal and external limiting membranes.
- Proliferation of neuroepithelial cells gives rise to a pseudostratified epithelium and the outermost cells move towards the centre of the cavity of the neural tube.
- 3 zones
- Outer most layer
- An acellular layer
- Becomes the white matter.
- Neurones in the mantle zone send axons into the marginal zone and outwards beyond the neural tube as motor fibres.
- Formed by Neuroblast
- Becomes
- Grey matter
- Basal ganglia
- Hippocampi.
- 4th week:
- proliferating neuroblasts in the mantle zone give rise to
- Basal plate
- Ventral thickenings
- Form motor neurones
- Corpus striatum
- begins as a thickening of the mantle zone of the basal part of the telencephalic vesicle.
- Later divided into medial and lateral parts by descending axons constituting the internal capsule.
- Remainder of the mantle zone of the telencephalic vesicle is thinner and is called the pallium.
- This becomes the cerebral cortex. In the medial wall of the pallium a thickening arises and bulges into the developing lateral ventricle. This gives rise to the hippocampus; the hippocampal sulcus separating it from the rest of the pallium
- Alar plate
- Dorsal thickenings
- separated by the sulcus limitans.
- Form sensory neurones
- The margins of the alar plates (the rhombic lip) give rise to the cerebellum
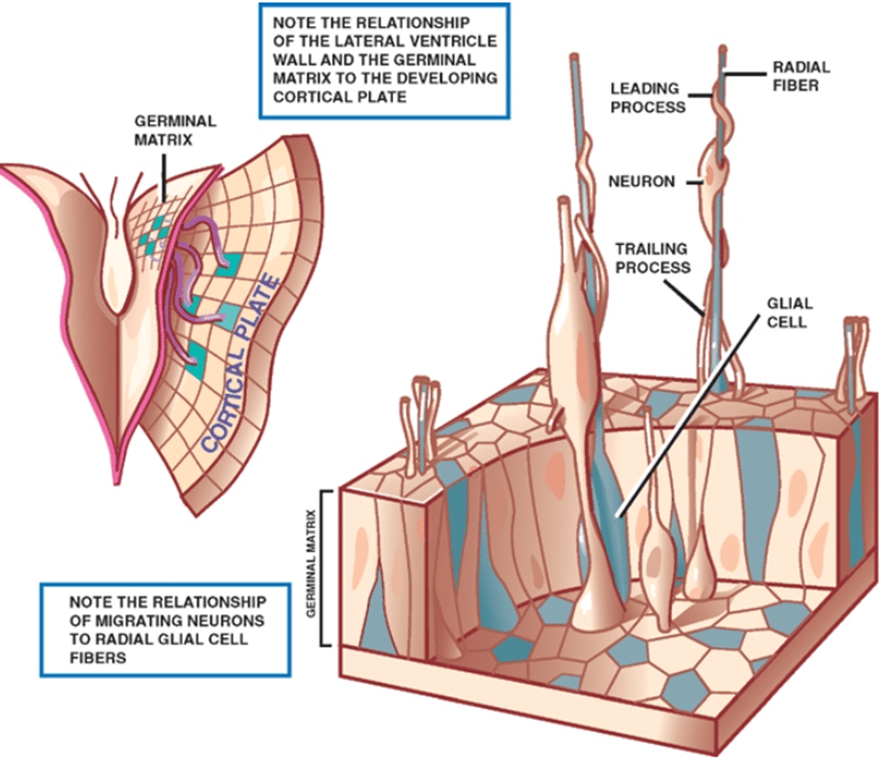
- Aka: germinal matrix (subependymal walls of the lateral ventricles)
- Innermost layer
- Site of mitotic division for both Neuroblast and Glioblast
- 2-phase process consisting of neuroblasts and Glioblasts.
- 1st phase
- Neuroblasts
- goes through its most rapid proliferation from 2 to 4 months of gestation.
- Neuroblasts are cells that will develop into nerve cells.
- Neuroblasts subsequently migrate outwards to form the Mantle zone
- 2nd phase
- Glioblasts
- Glioblasts are cells that will form the basic support structures in the mature brain.
- Astrocytes and oligodendrogliocytes form first
- Ependymal cells formed last
- Covering the developing ventricular system
- X Microglia are derived from mesenchyme (NOT PART OF GLIOBLAST)
- Undergo mitosis in the ventricular zone
- Is also occurring from 2 to 4 months of gestation, but it goes through its most rapid proliferation from roughly 5 to 12 months postnatally
- Both neuroblasts and Glioblasts begin to grow rapidly during proliferation, dividing and multiplying to create the number of nerve cells a person will have for life, approximately 100 billion.
Marginal zone
Mantle zone
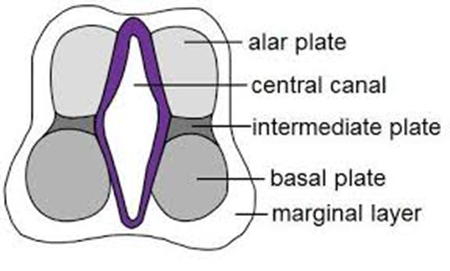
Ventricular zone
- Interruption of the 3rd stage of CNS development:
- Abnormally small brain size for the body (2 to 3 standard deviations below average) usually results (microcephaly)
- Usually associated with mild to moderate mental retardation.
4th stage: Migration
- 6 to 8 weeks of gestation through 8 months gestation.
- Movement of nerve cells from the proliferation zone (at the future site of the ventricular system) to their final position somewhere in the CNS.
- The movement of cells is thought to occur along migrational pathways, different groups of cells going in different locations.
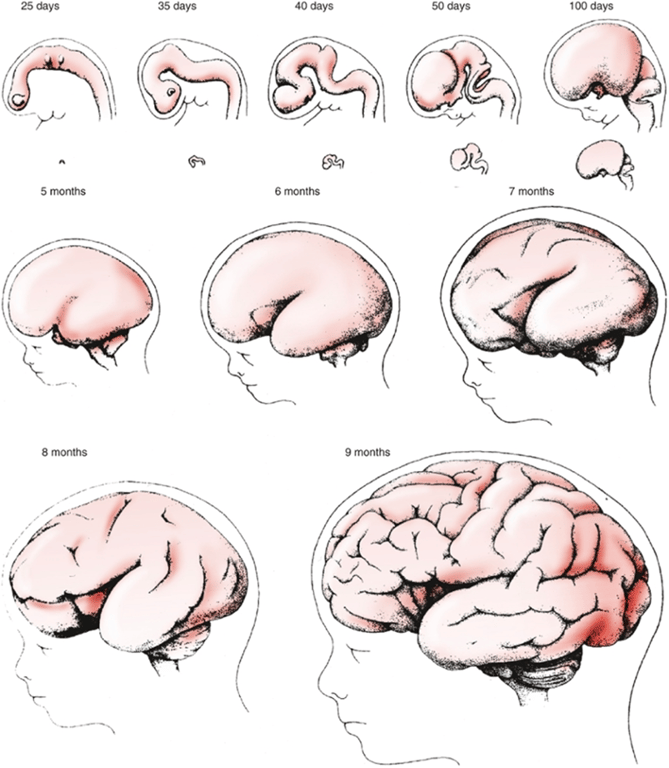
- For example, some cell will move to the outer surface of the cerebrum, forming the 3-mm-thick cerebral cortex (remember this from the second stage - ventral induction).
- Two methods of organization
- Migration begins with the innermost layer (layer 6 - this layer is present by week 8 of gestation) and subsequently progresses towards the outermost layer (layer 1 - present by the 8th month of gestation).
- The layers of the cerebral cortex are designed to either receive or send information.
- Cortical layer 5 is designed for control of body movement (sending motor commands)
- Cortical layer 4 takes in information from the senses.
- To complicate matters further the cerebral cortex is not only organized in layers (horizontally) but it is also organized in columns (vertically).
- Cells within a column will share a functional feature that is different from the functions of the cells in the column next to it.
- The process of migration can be thought of as an automated conveyer belt: (See Tumour staging)
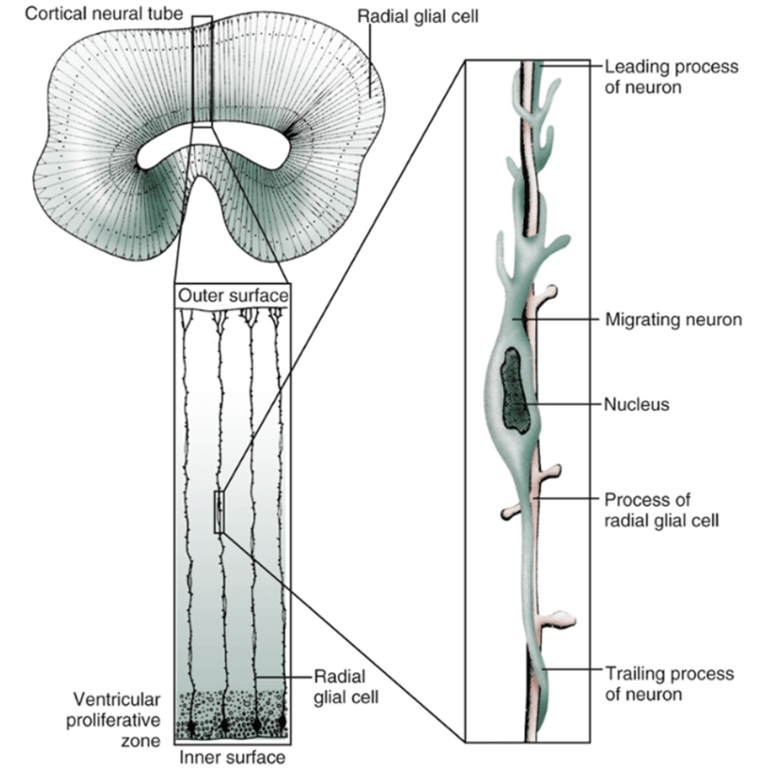
- First, a cell changes from a neuroblast to a neuron and becomes aligned to a radical glial cell.
- Facilitated by radial glial fibers that extend from the ventricular surface to the pia.
- These glia provide an avenue for neuronal migration, which follows an orderly, predictable sequence;
- Those neurons destined for the deepest cortical layer (layer 6) migrate early, followed by neurons that will form successively superficial cortical layers.
- An exception are the neurons that are destined to form the most superficial layer, the molecular layer (layer 1), which apparently migrate first.
- Second, the neuron cell propels itself along the surface of the glial cell.
- When neuron reaches its destination, the neuron is detached from the glial cell.
- During the process of migration
- Formation of convolutions occur (Groups of cells migrate to form gyri and sulci)
- Formation of corpus callosum,
- Formation of lobes (frontal, parietal, temporal occipital, limbic lobe)
- A minority of neurones migrate tangentially from the ganglionic eminence of the germinal matrix along axons rather than radial glial cells. These are destined to become GABAergic interneurons.
- Similar migratory processes are involved in the development of the cerebellar cortex and certain brain stem nuclei.
Layer organization: cerebral cortex contains 6 layers of cells.
Column organization
- Disruption in the migration of neurons
- Abnormal brain gyral patterns and collections of neurons in abnormal locations (called heterotopias).
- Children who are born prematurely are delivered during the later part of the migration period.
- Children who sustain brain injury during this period (for example, SEH - subependimal haemorrhage or PVL - periventricular leukomalacia) can have disorders or late migration.
- With these conditions → disruption of the radical glial fibers that then pull back from the cortical surface. → lack of glial fibres in guiding the neurons → neurons become stranded → Some researchers feel that disorders of late migration may contribute to the impaired coordination, visual perceptual problems, and seizures experienced by many children born prematurely who do not show evidence of other brain injury.
5th Stage: Organization
- Begins at 6 months of gestation and continues well after birth.
- Neurons begin to sprout axons and dendrites.
- At this stage most neurons consist of three parts:
- A cell body
- Axons
- Form 1st
- Axon sprouting begins during neuronal migration.
- Involves formation of short and long axons for nearby and distal targets, respectively.
- nerve fibers that send signals away from the cell body to other neurons.
- Neurons usually only have one axon.
- Dendrites
- Form 2nd
- a number of short tree-like branches that receive signals from other neurons.
- A neuron can have hundreds of dendrites.
- Many more neurones are formed than ultimately comprise the mature brain;
- Synaptogenesis
- Form 3rd
- The synapse permits the conduction of electrochemical impulses among a large number of neurons almost simultaneously.
- Initially, there are more synapses created than are needed; however, only those used will survive. Just as we prune a tree that is overgrown, the brain "prunes" away synapses that are not used.
- This process is a way of fine tuning the maturing CNS.
- Just like with neurons, the brain starts out creating more synapses than it will ever need. Over time (and into the teen years) those cells that are not needed will die off.
- Apoptosis is an important aspect of brain development. It generally coincides with synaptogenesis.
- It is important to note that the brain is not rigid and inflexible even after the teen years.
- Adults can still make new synaptic connections for novel learning experiences.
6th Stage: Myelination
- Begins at 6 months of gestation and continues into adulthood
- Location
- Spinal nerve roots
- Some cranial nerves
- Sme longitudinal tracts of the brainstem
- The glial cells produce myelin rapid impulse transmission in the cerebral medulla forming white matter.
- True maturity of the CNS only occurs after the Myelination process has fully developed.
- Disruption
- Chronic hypoxia in premature infants is probably the most common cause of delayed myelination and contributes to delays in clinical neurological maturation.
- Unlike disorders of neuroblast migration, delay in myelination is not necessarily irreversible, and if the insult is removed, the process may catch up to the appropriate level of maturity over time.
- Takes long time: 16wks to 30 yrs
Pathway | Begins | Completed |
FETAL ONSET | ㅤ | ㅤ |
Spinal motor roots | 16 wk | 42 wk |
Cranial motor nerves III, IV, V, VI | 20 wk | 28 wk |
Spinal sensory roots | 20 wk | 5 mo |
Medial longitudinal fasciculus | 24 wk | 28 wk |
Acoustic nerve | 24 wk | 36 wk |
Ventral commissure, spinal cord | 24 wk | 4 mo |
Trapezoid body and lateral lemniscus | 25 wk | 36 wk |
Inferior cerebellar peduncle (inner part) | 26 wk | 36 wk |
Inferior cerebellar peduncle (outer part) | 32 wk | 4 mo |
Superior cerebellar peduncle | 28 wk | 6 mo |
Middle cerebellar peduncle (pontocerebellar) | 42 wk | 3 yr |
Habenulopeduncular tract | 28 wk | 34 wk |
Dorsal columns, spinal cord | 28 wk | 36 wk |
Ansa reticularis | 28 wk | 8 mo |
Medial lemniscus | 32 wk | 12 mo |
Optic nerve | 38 wk | 6 mo |
Corticospinal (pyramidal) tract | 38 wk | 2 yr |
Optic radiations (geniculocalcarine) | 40 wk | 6 mo |
Acoustic radiations (thalamocortical) | 40 wk | 3 yr |
POSTNATAL ONSET | ㅤ | ㅤ |
Fornix | 2 mo | 2 yr |
Thalamocortical radiations | 2 mo | 7 yr |
Corpus callosum | 2 mo | 17 yr |
Ipsilateral intracortical association fibers, frontotemporal and frontoparietal | 3 mo | 32 yr |
Mamillothalamic tract | 8 mo | 6 yr |