General
- Most commonly found malformation of cortical development in the surgical series
- Accounts for approximately 20-50% of all cases that have undergone epilepsy surgery.
- Attempts at classifying FCD have been incomplete and unsatisfactory because of the absence of a scientifically proven aetiology.
- A frequent cause of refractory epilepsy.
Classification
- Many-Blumcke most commonly used
- Current 3 tier ILAE classification system for Focal cortical dysplasia (FCD)
- Please note that the rare association between FCD type IIa and IIb with hippocampal sclerosis, tumors, or vascular malformations should not be classified as FCD type III variant.
ㅤ | ㅤ | ㅤ | ㅤ | ㅤ |
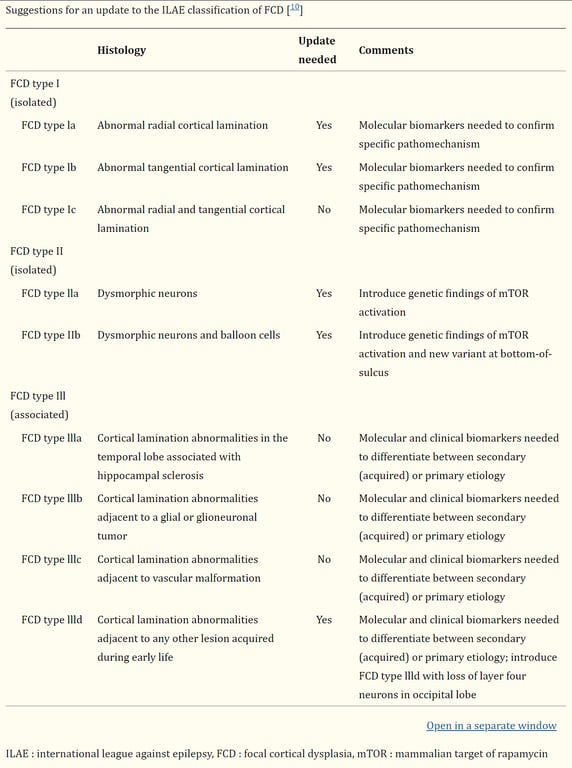
FCD type I (isolated) | Focal cortical dysplasia with abnormal radial cortical lamination (FCD type Ia) | Focal cortical dysplasia with abnormal tangential cortical lamination (FCD type Ib) | Focal cortical dysplasia with abnormal radial and tangential cortical lamination (FCD type Ic) | ㅤ |
ㅤ | ㅤ | ㅤ | ㅤ | ㅤ |
FCD type II (isolated) | Focal cortical dysplasia with dysmorphic neurons (FCD type IIa) | ㅤ | Focal cortical dysplasia with dysmorphic neurons and balloon cells (FCD type IIb) | ㅤ |
ㅤ | ㅤ | ㅤ | ㅤ | ㅤ |
FCD type III (associated with principal lesion) | Cortical lamination abnormalities in the temporal lobe associated with hippocampal sclerosis (FCD type IIIa) | Cortical lamination abnormalities adjacent to a glial or glioneuronal tumor (FCD type IIIb) | Cortical lamination abnormalities adjacent to vascular malformation (FCD type IIIc) | Cortical lamination abnormalities adjacent to any other lesion acquired during early life, e.g., trauma, ischemic injury, encephalitis (FCD type IIId) |
ㅤ | Temporal lobe | Tumour | Vascular | Congenital |
ㅤ | ㅤ | ㅤ | ㅤ | ㅤ |
FCD type III (NOS) : if clinically/radiologically suspected principal lesion is not available for microscopic inspection. | ㅤ | ㅤ | ㅤ | ㅤ |
ㅤ | ㅤ | ㅤ | ㅤ | ㅤ |
Kim et al 2019 has a suggestion for an updated version of the ILAE system
Mech
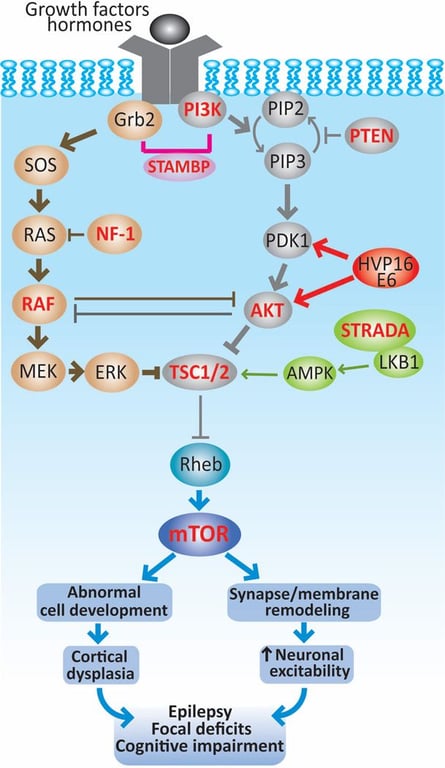
- The mTOR pathway can become hyperactive (thick lines) by mutations of genes encoding upstream regulators (TSC1, TSC2, STRADA, PTEN or NF-1), components of the pathways that converge on TCS1/2, such as RAF, AKT, PI3K and also of mTOR (highlighted in red).
- The recent discovery of HPV16 E6 in FCD type IIa and IIb along with other DNA viruses suggest that an infectious etiology can contribute to the pathogenesis of this condition.
- Together, activated mTOR pathway results in abnormally increased cell growth and proliferation that could account for the anatomical lesions encountered in cortical dysplasias.
- In addition, enhanced mTOR signaling can modify the expression of neurotransmitter receptors and ion channels, which can change membrane properties and synaptic organization leading to neuronal hyperexcitability.
- Both morphological and functional alterations at the cellular and circuit levels may lead to epilepsy, focal deficits and cognitive dysfunction in patients with cortical dysplasias.
Radiology
- MRI
- General features of focal cortical dysplasia include:
- Cortical thickening
- Blurring of white matter-grey matter junction with abnormal architecture of subcortical layer
- T2/FLAIR signal hyperintensity of white matter with or without the transmantle sign
- T2/FLAIR signal hyperintensity of grey matter
- Abnormal sulcal or gyral pattern
- Segmental and/or lobar hypoplasia/atrophy
- There is no oedema, calcification, or contrast enhancement
- Also, each type of focal cortical dysplasia can exhibit more or less of these features.
- Variation on MRI according to Blumcke classification of focal cortical dysplasia.
- location
- Type Ia:
- Confined to temporal lobes
- if associated with hippocampal atrophy (as is common), it is now classified as type IIIa in the Blumcke classification
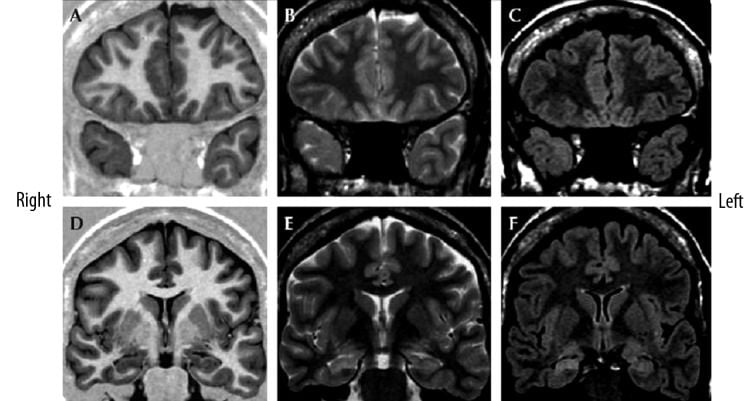
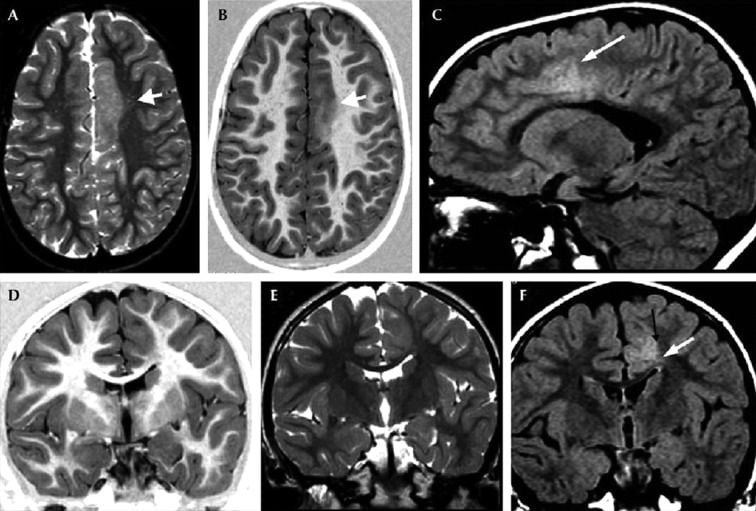
- Focal cortical dysplasia type Ia with ipsilateral hippocampal sclerosis (“dual pathology”) in a 31-year-old female by Blumcke IIIa.
- Coronal MR images:
- T1-weighted (A,D),
- T2-weighted (B,E)
- FLAIR T2-weighted (C,F)
- Hypoplasia of the right temporal pole with volume loss of the white matter which leads to mild hyperintensity on T2-weighted images
- Mild blurring of the cortical-white matter junction is visible on both T1- and T2-weighted images (A–C).
- The right hippocampus is atrophic, with decreased signal on IR-T1W images and increased signal on T2W images, consistent with hippocampal sclerosis.
- Type Ib:
- More frequently seen outside of the temporal lobes
- structure
- blurring of grey/white matter junction (less marked than with Type II FCD)
- prominent segmental or lobar atrophy/hypoplasia with loss of regional white matter volume
- signal
- white matter
- moderately increased T2/FLAIR signal
- decreased T1 signal
- Location
- Commonly found in frontal lobes
- Less likely to be in the temporal lobes compared to Type I FCD
- Structure
- Abnormal gyri and sulci
- Marked blurring of grey/white matter junction
- Cortical thickening
- Signal
- White matter
- moderately increased T2/FLAIR signal, typically brighter than the adjacent cortex
- decreased T1 signal
- focal signal abnormality may extend from cortex to ventricle (transmantle sign):
- not seen in type I
- Grey matter
- some increase in T2 signal
- despite an increase in T2 signal, the cortex remains hypointense to much brighter adjacent white matter
- more evident than in type I
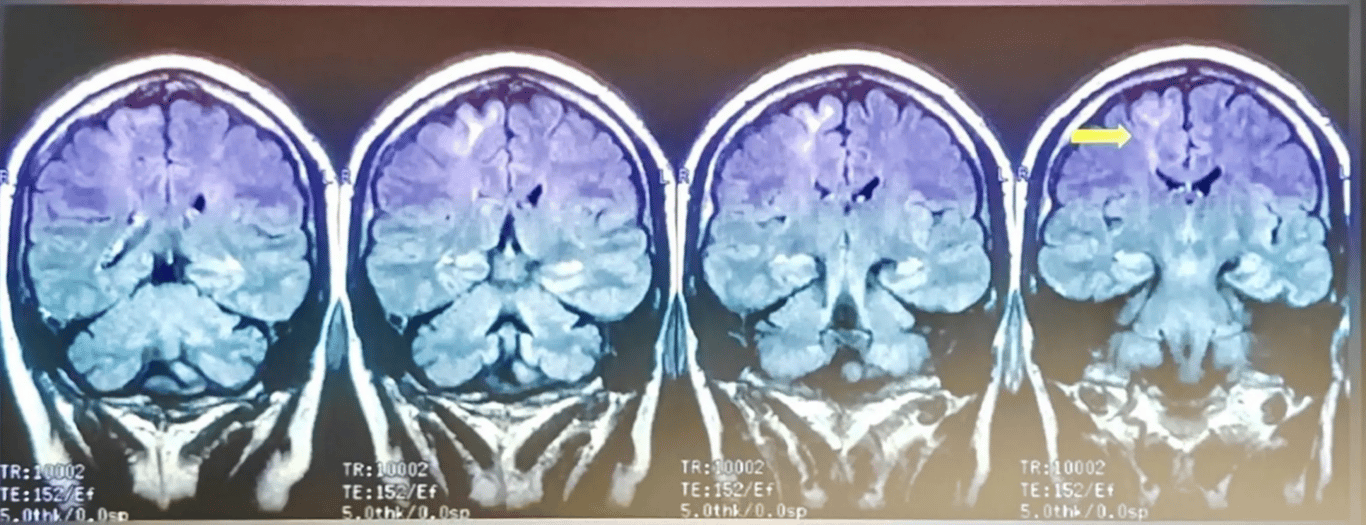
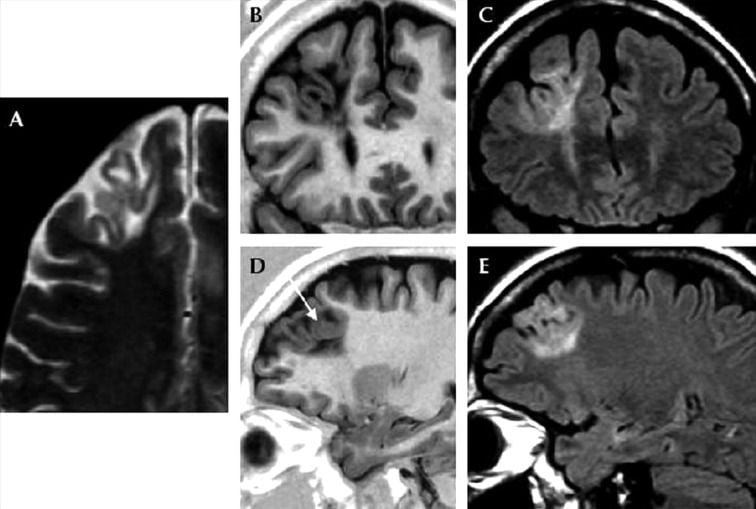
- Focal cortical dysplasia type IIb of the left frontal cortex in a 9-year-old female.
- Key
- Transverse TSE T2WI (A) and transverse TSE IR T1WI (B).
- Sagittal TSE FLAIR T2WI (C).
- Coronal TSE IR T1WI (D), coronal TSE T2-WI (E) and coronal TSE FLAIR T2WI (F).
- Thickening of the left paramedian frontal cortex which shows a blurred demarcation with the white matter both on T1W and T2W either transverse and coronal images (white arrows, A,B,D,E). On FLAIR coronal sequence (F) the junction between GM/WM seems to be more defined (black arrow), contrary to what is observed most frequently. The hyperintensity of the WM, extending toward the ventricle (transmantle sign) is better appreciated on FLAIR sequences (white arrows, C,F).
- Focal cortical dysplasia type IIb of the right frontal cortex in a 41-year-old female.
- Key
- (A) Transverse TSE T2WI (magnification)
- (B) Coronal TSE IR T1WI
- (C) Coronal TSE FLAIR T2WI
- (D) Sagittal TSE IR T1WI
- (E) Sagittal TSE FLAIR T2WI
- Abnormal gyration of the left frontal cortex which presents very sharp demarcation with the white matter both on T1W and T2W images.
- Pronounced increased signal of the subcortical white matter on T2WI which tapers toward the ventricle (transmantle sign).
- The dysplastic cortex appears to be of normal thickness in (A) and (B). In (D), focal thickening of the cortex seems to be present (arrow), probably due to convolution of gyri.
- Associated with adjacent other abnormalities
- Imaging appearances will be dominated by the associated abnormality rather than the dysplasia itself.
Type I
Imaging
Type II
Imaging
Type III
Treatment and prognosis
- Surgical resection of the refractory epileptogenic area of focal cortical dysplasia typically leads to good seizure control.
- In the presence of transmantle sign better post-surgical outcomes have been reported.