General
- There is no uniformly accepted nomenclature for malformations characterized by duplicate or split spinal cords.
- Pang et al have proposed the following.
- The term split cord malformation (SCM) should be used for all double spinal cords, all of which appear to have a common embryologic aetiology.
Definition
- all double spinal cords that may be separated by a dural fibrous band or by bone.
Embryology
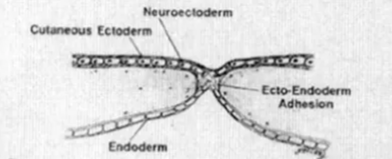
- Normal
- Bilaminar s(x)
- Epiblast: Adjacent to Amnion
- Hypoblast: adjacent to Yolk sac
- Caudal midline cells form primitive streak
- Rostral midline cells form primitive knot (Hensen's node)
- Epiblast cell adjacent to the primitive streak migrate through the primitive streak and form the mesoderm
- Now there is a trilaminar embryo
- Day 16
- Primitive streak begins to regresses so that the primitive knot occupies a progressively more caudal position within the embryonic disc.
- At the same time, cells situated along the rostral lip of the primitive knot migrate between the epiblast and hypoblast and are gradually laid down as the notochordal process
- Notocord is then canalized to form a tube that is now in communication with the amnion through the primitive pit.
- Gastrulation ends
- Formation of Primitive neurenteric canal:
- Canalized notochordal process fuses with and opens through the underlying endoderm (intercalation) to complete a temporary passage through the embryo, which now joins the amnion and yolk sac.
- Exist of 2-8 days
- Final fate:
- open notochord separates from the endoderm (excalation) and reforms a blind tube, thereby permanently obliterating the primitive neurenteric canal and the communication between the amnion and yolk sac
2nd wk
2-3 wk process of gastrulation starts
3wk
- Abnormal
Pathology
- Unified theory for the development of both types of SCM by Pang et al
- An ontogenetic error occurred
- When the primitive neurenteric canal closes
- Formation of an accessory neurenteric canal though the midline embryonic disc
- Maintaining communication between ectoderm and endoderm within the canal
- This abnormal fistula --> regional splitting of the notochord and the overlying neural plate
- The rest of the notochord is well formed and inducing the neural plate to roll up through the ascension of the neural crest cells to form the neural tube
- Endomesenchymal tract formation
- The abnormal fistula matures with the surrounding mesenchyme condensing around it + a "pinch" of endoderm protruding from the base of the fistula,
- occupies the space between the split notochord and split neural plate
- Mesenchyme has pluripotent ability: can form fibroblasts, cartilage, bone, blood vessels, fat, and myoblasts
- The normal neurenteric canal has no Notochord splitting so does not produce a split cord malformation
- Located
- Variable
- Lie rostral to the primitive neurenteric canal because the primitive pit into which the normal neurenteric canal opens, ultimately comes to lie opposite the coccyx. all known SCMs involve cord segments that are rostral to the coccyx.
- Due to healing ability of the embryo:
- The split notochord heals hence rarely see a bifid vertebral bodies
- The endomesenchymal tract connecting the Gut to Skin rarely fully persist but remnants parts of the original tract remain in modified forms
- Hypothetical steps in the formation of the endomesenchymal tract.
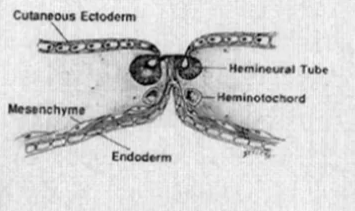
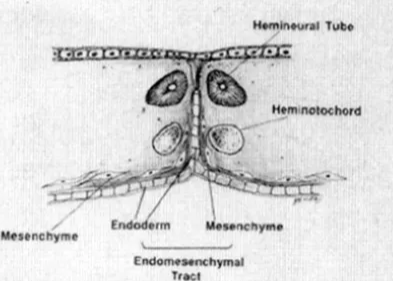
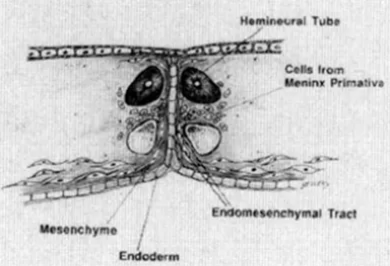
If meninx primativa cells surround both cord it will be type 2 if it surround each cord will be type 1
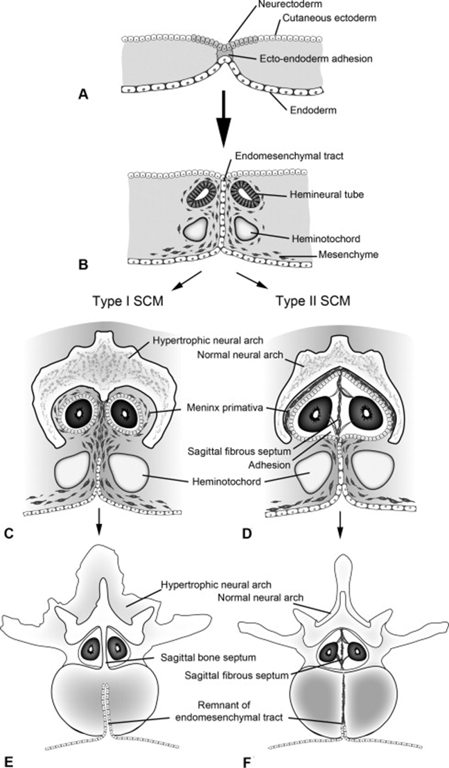
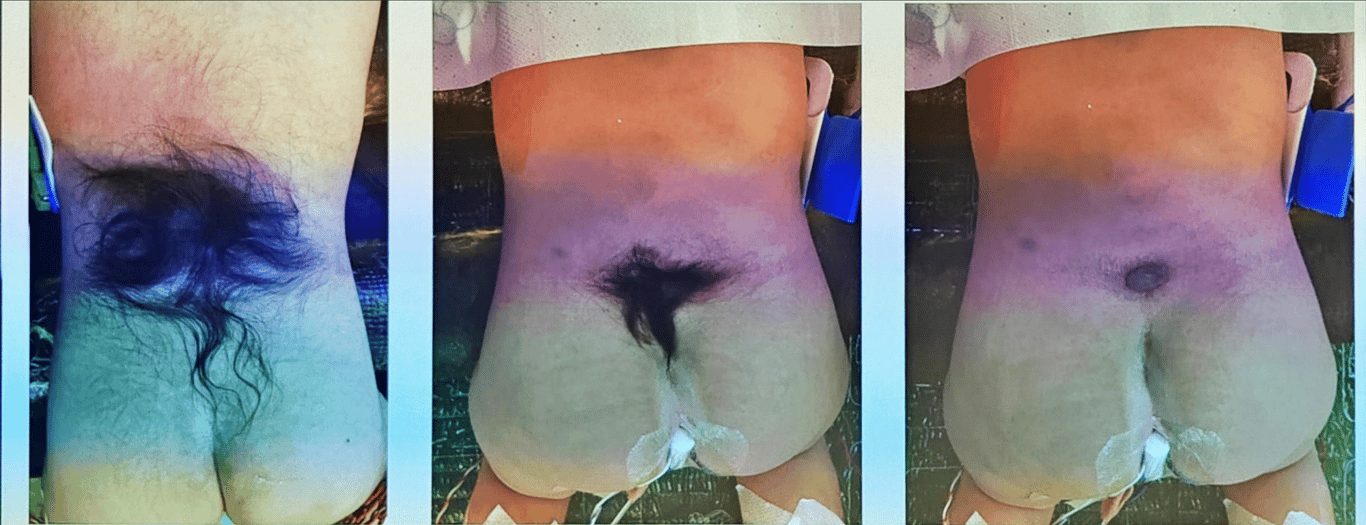
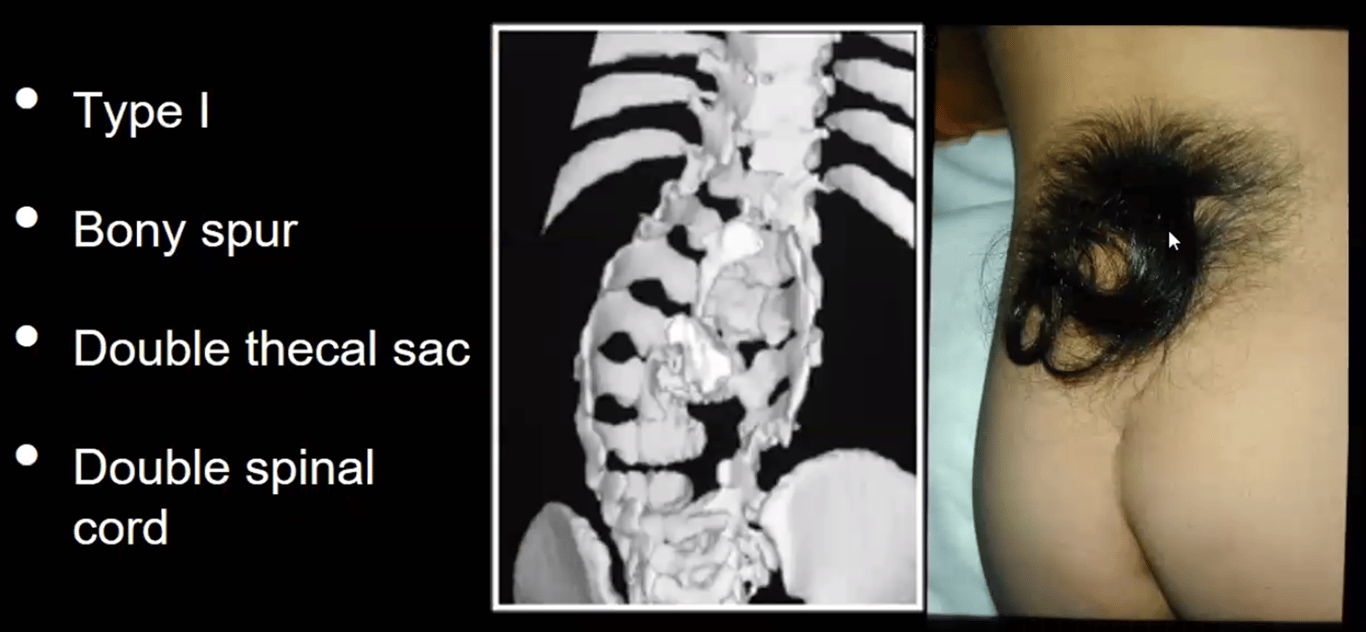
Anomalies of the overlying skin such as hypertrichosis (A, C) may signify the presence of an underlying anomaly.
(A) Abnormal connections with the overlying skin can be seen, referred to as dermal sinus tracts. Similarly abnormal connections can arise anteriorly, connecting the spine with the lumen of the gut or respiratory tract. Myelomeningocele manqué
(B) is a similar anomaly in which blood vessels and nervous cells form a midline septum. Several tumours are also believed related to this abnormal development (C).
Dermoid cysts and lipomas are two such tumours, and they are frequently found posterior to the spinal cord and may be associated with a split cord malformation, as in this case.
Clinical features
Types
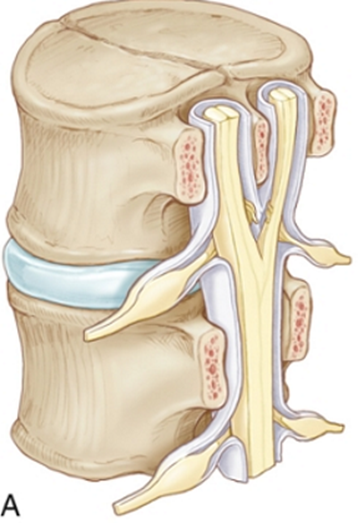
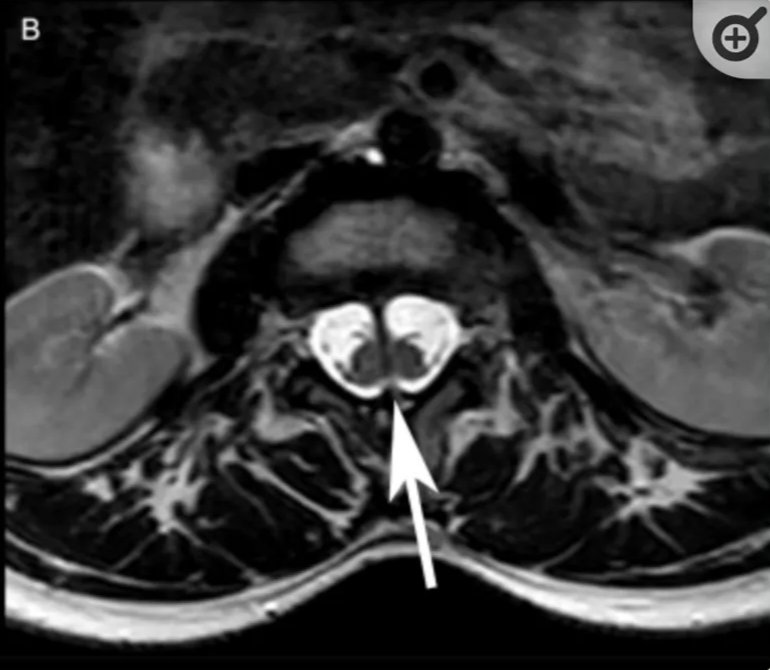
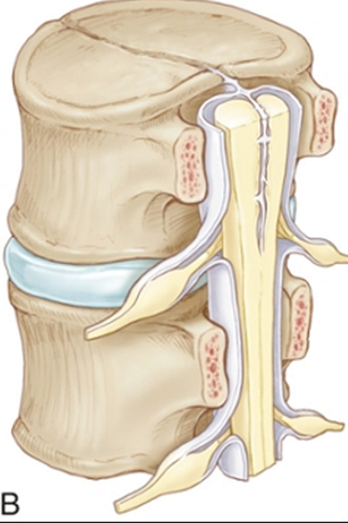
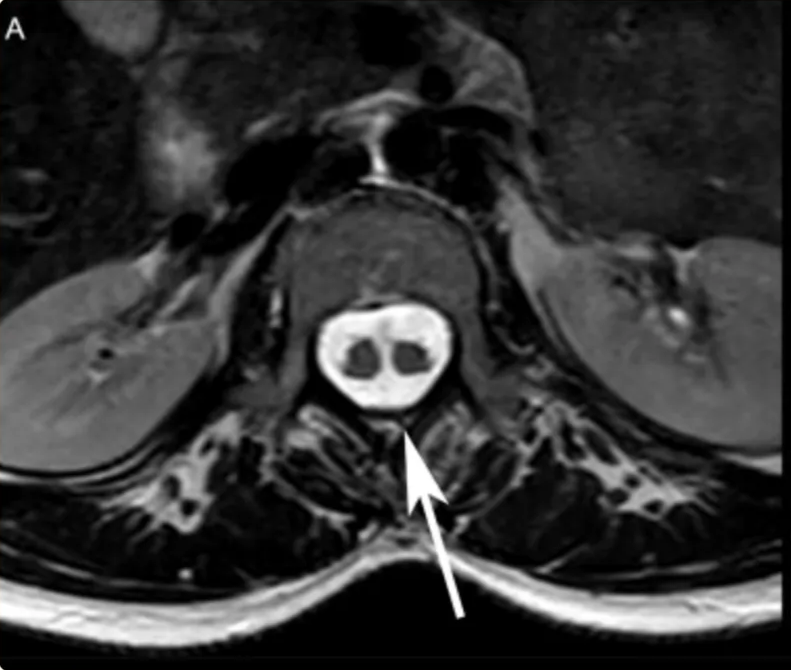
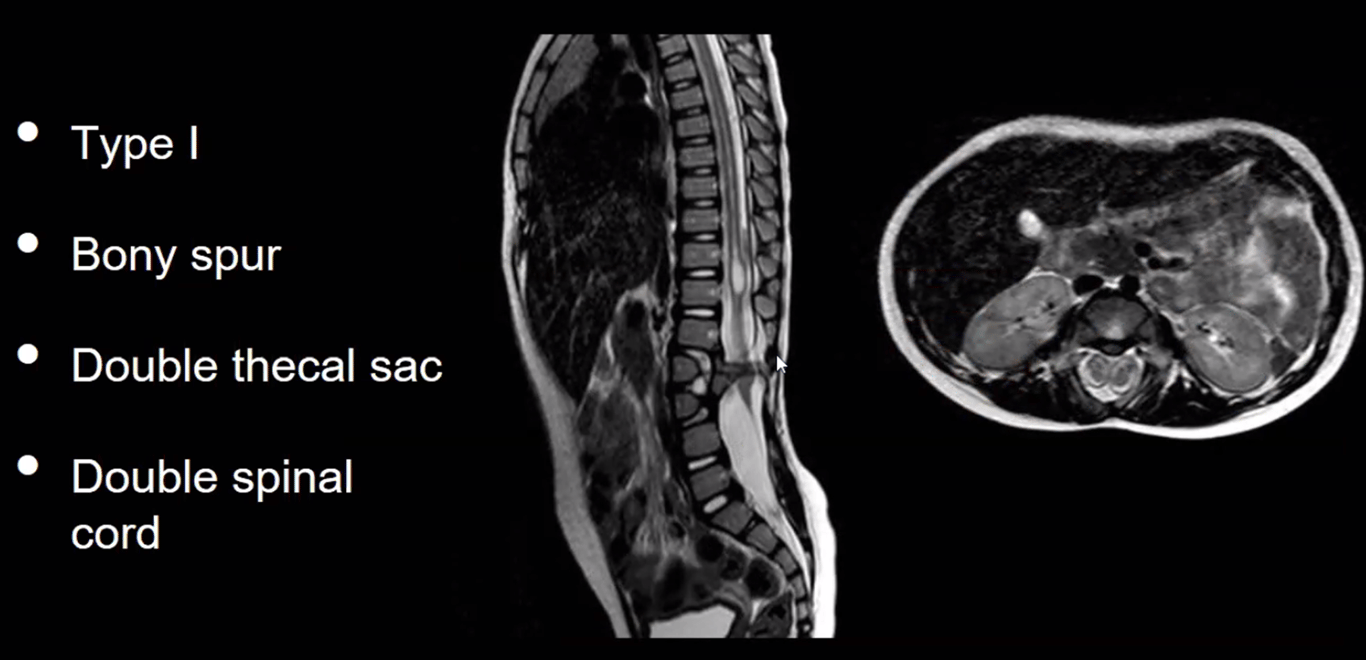
SCM Type | 1 (Diastematomyelia) ONE=BONE | 2 (Diplomyelia) |
Anatomical features | -Two hemicords in two dural sleeves -Separated by a midline bony spur. -Hypertrophic laminae are often fused to adjacent levels. | -Two hemicords in a single dural sleeve. -Separated and tethered by a fibrous band attached to the dura. -Each hemicord has nerve roots arising from it. -Usually no spine abnormality at the level of the split -usually spina bifida occulta in the lumbosacral region |
Clinical presentation | -2/3 overlying skin abnormalities (nevi, hypertrichosis (tuft of hair), lipomas, dimples, or haemangiomas) -Orthopaedic foot deformity (neurogenic high arches). | |
Radiographic | CT - Bony spur - intersegmental fusion and adjacent spina bifida are virtually pathognomonic. - Other abnormalities of spine at the level of the split (dorsal hypertrophic bone where the median “spike” attaches MRI - two hemicords in separate subarachnoid spaces. | Two hemicords seen within a single subarachnoid space on T2-weighted MRI. High-resolution T2-weighted MR or CT myelography may show fibrous septum attached to the dura. |
Location | Typically lumbar | May occur anywhere along the spinal axis |
Treatment | - Symptoms are most commonly due to tethering of the cord; and are usually improved by untethering. - The bony septum must be removed and the dura reconstituted as a single tube - These spines are often very distorted and rotated, and therefore start at normal anatomy and work towards defect. - ✖ DO NOT cut the tethered filum until after the median septum is removed to avoid having the cord retract up against the septum. | - Consists of untethering the cord at the level of the spina bifida occulta, and occasionally at the level of the split. - More favourable and not necessary need treatment |
- Type 1 sub types: Mahapatra classification
Subtype | Spur Location | Frequency (%) | Syringomyelia Incidence | Neurological Deterioration Risk | Surgical Complexity | Clinical Outcomes |
Type Ia | Center of split - equally duplicated cord above and below spur | 50% | 7% | 0% | Standard | Excellent - no deterioration reported |
Type Ib | Superior pole - no space above, large duplicated cord below | 17% | 80% | 0% | Standard | Good - no neurological worsening |
Type Ic | Lower pole - large duplicated cord segment above | 13% | 75% | 0% | Standard | Favorable - no postoperative deterioration |
Type Id | Straddling bifurcation - extends entire length of split | 20% | 100% | 67% | High Risk | Guarded - highest complication rate |
Images
Surgical techniques (Wang 2017)
Indication for Surgery
- Type 1 Split Cord Malformation (with an Osseous Divide (SCM-OD))
- Tethered Cord Syndrome, which is a primary indication for surgery.
- Urination or defecation disorders.
- Weakness in both lower extremities.
- Talipes equinus (foot deformity).
- Asymptomatic patient but has scoliosis that requires orthopaedic surgery.
- This is because correcting the scoliosis will stretch the spinal column, and the fixed osseous divide would cause traction and potential injury to the spinal cord.
- Most experts believe that if a patient with scoliosis is found to have an SCM-OD, the osseous divide should be surgically removed first, with scoliosis correction following 2–6 months later.
- Early surgery is particularly important for children and adolescents in their growth and development period to prevent secondary damage caused by the growth of the spinal cord or daily activities.
Surgical Technique
- Preoperative:
- Localization
- CT-guided positioning is used to accurately locate the osseous divide.
- The patient is placed in a prone or lateral position on a CT bed, and a needle is guided to the spinous process, which is then marked with an injection of methylthioninium chloride.
- IOM
- Anaesthesia and Positioning:
- The procedure is performed under general anaesthesia with intubation.
- The patient is placed in a prone or lateral position on the operating table, positioned so the osseous divide is vertical to the bed to facilitate the operation.
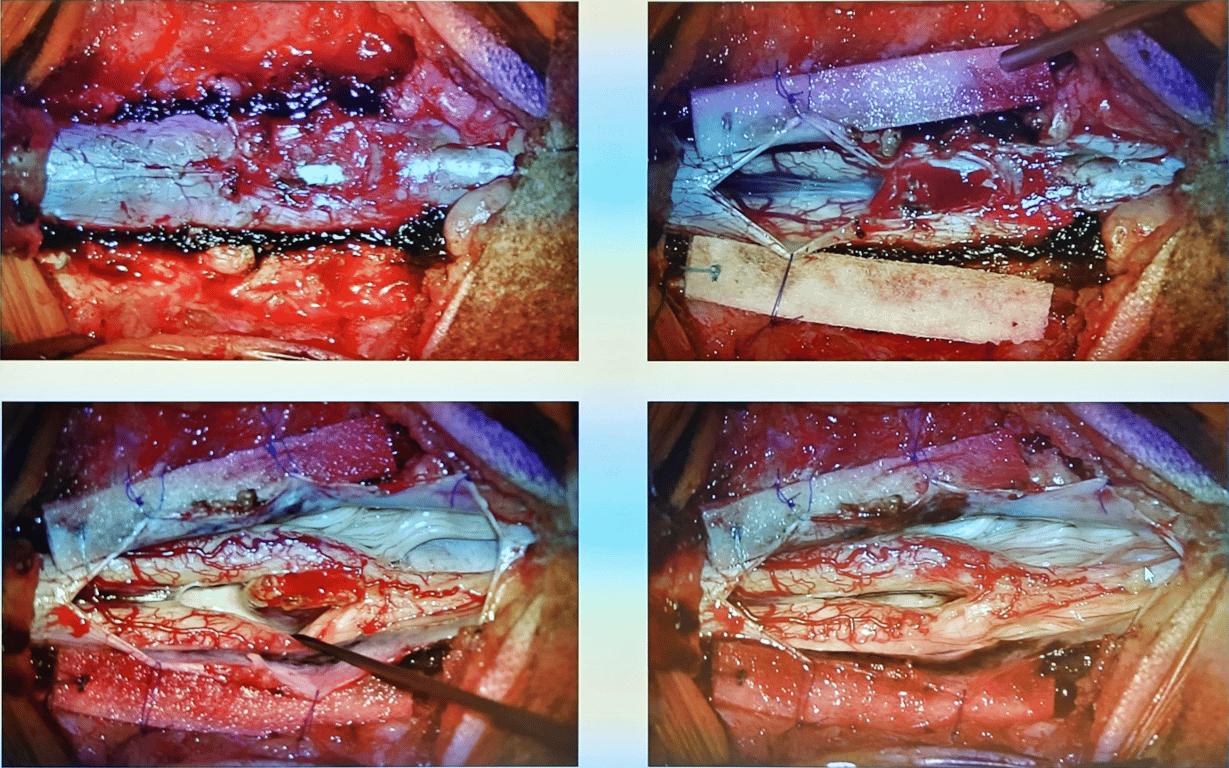
- Resection of the Osseous Divide (OD):
- Blood vessel on the spur is coagulated
- The osseous divide and the spinal canals above and below it are exposed.
- The OD is resected using tools such as rongeurs, arc bone chisels, or a high-speed drill.
- A wide brain spatula may be used to shield the dura mater and spinal cord from injury, especially when using a grinder.
- Care is taken to preserve the integrity of the spinal dura mater during the resection.
- Bleeding from the base of the divide, which is vertebral cancellous bone, is managed with bone wax or hemostatic sponge.
- Chase spur up to ventral dura where spur may widen beyond vision
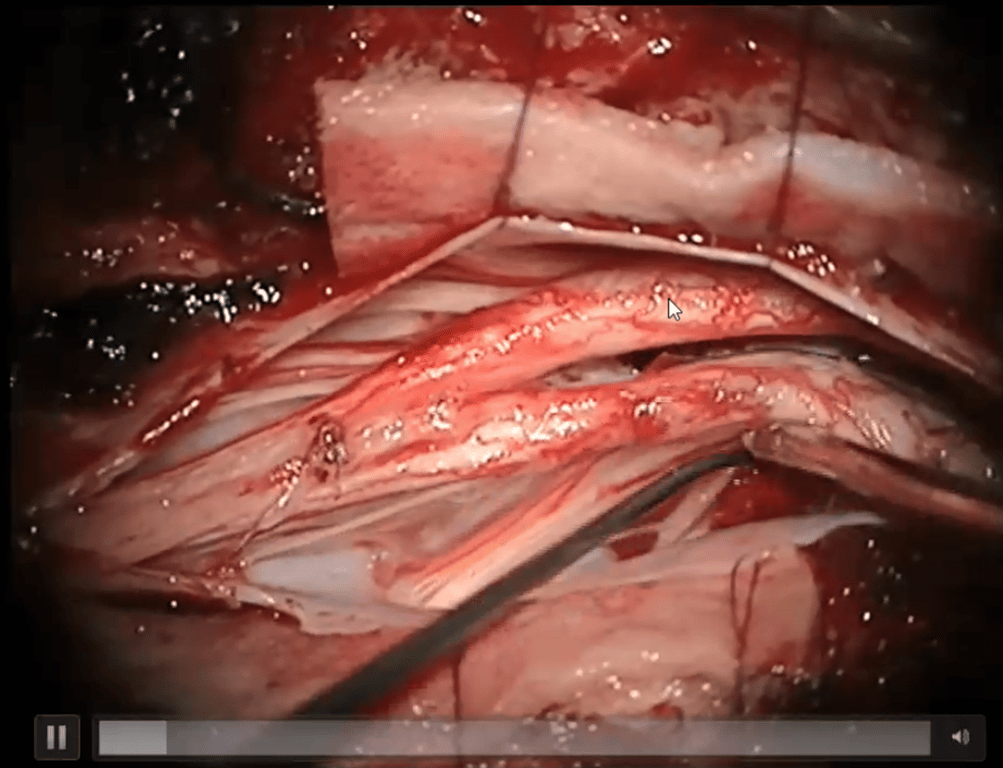
- Dural Sac Moulding:
- After the divide is removed, the dural mater of both spinal cords is cut open to relieve any membrane compression.
- Medial roots of the cords are sacrificed
- The dura is then sutured to create a single dural cavity containing both semi-spinal cords. The dorsal (back) side is continuously sutured, while the ventral (front) side is intermittently sutured.
- Addressing Associated Conditions:
- If the patient also has a tethered cord or thickened filum terminale, the filum terminale is cut microscopically to release the tethering.
- Other coexisting conditions like syringomyelia or congenital tumours and cysts are also surgically treated at the same time.
Outcomes of Surgery
- Overall Surgical Success:
- "good results" in a study of 142 patients, with no serious complications such as spinal cord or nerve injury.
- Postoperative CT scans confirmed complete resection of the osseous divide in all 142 patients, successfully relieving compression on the spinal cord.
- Symptom Improvement:
- Partial relief was achieved in patients with symptoms like urination-defecation disorders, muscle strength subsidence, Pes Cavus (high-arched foot), and toe movement disorders.
- 28 patients experienced relief from preoperative dry stool symptoms
- 12 patients with poor toe motion had significantly improved fine motor functions.
- Neurological Status and Complications:
- During a median follow-up of 35 months, no new permanent neurological damage was observed.
- Some patients experienced temporary numbness or pain on the surgery side, which typically resolved within 1–2 weeks.
- Three patients experienced a temporary decrease in lower limb muscle strength, which fully recovered within three months.
- Foundation for Future Scoliosis Treatment:
- The surgery creates good conditions for subsequent scoliosis correction.
- By removing the osseous divide, the surgery clears the way for a later orthopaedic procedure and avoids traction injury to the spinal cord during scoliosis correction.
- In the study, 109 patients underwent scoliosis correction 2–6 months after this initial surgery.