Intrathecal Baclofen
Indication
- If on oral baclofen doses that are high enough to cause side effects
- Global spasticity
- Spasticity due to
- Spinal cord lesion
- Painful spasms are present
- Due to advanced multiple sclerosis or after spinal cord injury
- Physical therapy and rehabilitation do not succeed in preventing harmful spasticity from appearing.
- Brain stem lesions
- Cerebral lesion
- Cerebral palsy patients
- Adult > children
- If able to walk, adequate doses (i.e., ones effective on the excess of tone that do not produce motor weakness) are difficult to find.
- Dystonia
- Positive test dose: + >1 Ashworth improvements
- Done under GA or LA, with either
- Direct injection of 50– 100 µg of baclofen via lumbar puncture, OR
- Insertion of a lumbar catheter to allow serial test doses or a test infusion to be undertaken.
- Aims of the test dose
- Assess the benefits of the treatment
- To examine for possible side effects.
- Patients with dystonia, however, may not experience much benefit from the brief test dose because they tend to get more benefit from the sustained effects of prolonged infusion.
CI
- ? Renal
- Hepatic disease
- Epilepsy
- Mental disease
- ? Gastrointestinal disease
- Baclofen can cause nausea, vomit, constipation
Pros
- IT baclofen fewer side effects due to direct administration and smaller dosage.
- This is because the GABAB receptors in the spinal cord are in the surface layers, allowing the baclofen to work directly.
- However, in the brain, the GABAB receptors are in the deeper layers, meaning there are fewer unwanted central effects.
ITB therapy components
- Externally- adjustable programmable pump system, containing a
- A drug reservoir (10– 40 ml),
- A battery,
- The drug delivery mechanism.
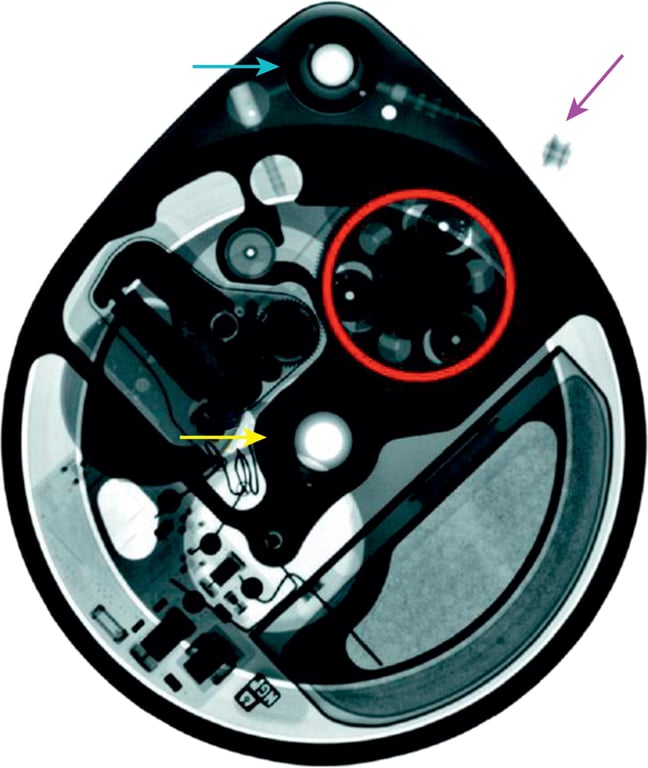
- Parts of a baclofen pump (Medtronic Synchromed)
- Consist of pump roller (red ring),
- Pump reservoir port for filling (yellow arrow),
- Catheter access port (CAP) (blue arrow)
- Pump-catheter connector (purple arrow).
Surgical tech
- Lateral position, right- side- up.
- Pump implantation
- Pump is implanted in a pocket in the right anterior abdominal wall.
- Avoided left side due to gastrostomies
- The pump pocket is either
- Subcutaneous OR
- Superficial to the rectus fascia
- Subfascial
- Deep to the fascia of the rectus and external oblique muscles
- Useful for smaller, thinner children
- A small incision is made in the lumbar region and the intrathecal catheter is inserted via a lumbar puncture with a Toohey needle.
- The tip of the catheter is placed at the level corresponding with the therapeutic indication:
- T10–T12 for spastic diplegia,
- C5–T2 for spastic tetraplegia,
- C1–C4 for generalized secondary dystonia.
- Tip position can be guided with X- ray
- Controversial: a higher tip position might be required for upper limb spasticity/ dystonia
- Some studies have suggested that CSF pulsatile flow is less in the thoracic and lumbar spine, with a consequent reduction in baclofen diffusion for catheters placed in this location
- Catheters can be inserted into the ventricle to allow intraventricular baclofen infusion
- Once the catheter is inserted to the desired position, the catheter is tunnelled through to the abdominal pocket, secured to the pump and the system is then implanted
- After implantation, the pump is activated and the system can start delivering baclofen immediately.
- Dose delivery
- The dose is gradually titrated upwards
- Usually with increments of 10% every 1– 2 weeks.
- Can have flexible dose programming delivering different amounts of baclofen at different times of the day.
- The pump reservoir needs to be emptied and refilled every 2– 6 months depending on the infusion rate and the drug concentration.
- The system will also need surgery to replace the pump when the battery is nearing end of life.
Dosing
- For patients with chronic, nonprogressive neurologic conditions (e.g., spinal cord injuries, stroke), ITB dosing should be relatively stable during the maintenance phase of therapy (1–3).
- Individuals with progressive diseases (e.g., amyotrophic lateral sclerosis or multiple sclerosis) may require frequent evaluation and dose adjustments
Risks and unwanted effects of ITB therapy include
- The risks of the surgery itself,
- Risks associated with pump system failure
- Problems with the refill procedures
- Calculate how much baclofen is used in a day for the patient
- Calculate how much is in a ml of baclofen
- You can then see if you loose a drop of baclofen can = one day loss of baclofen dosage
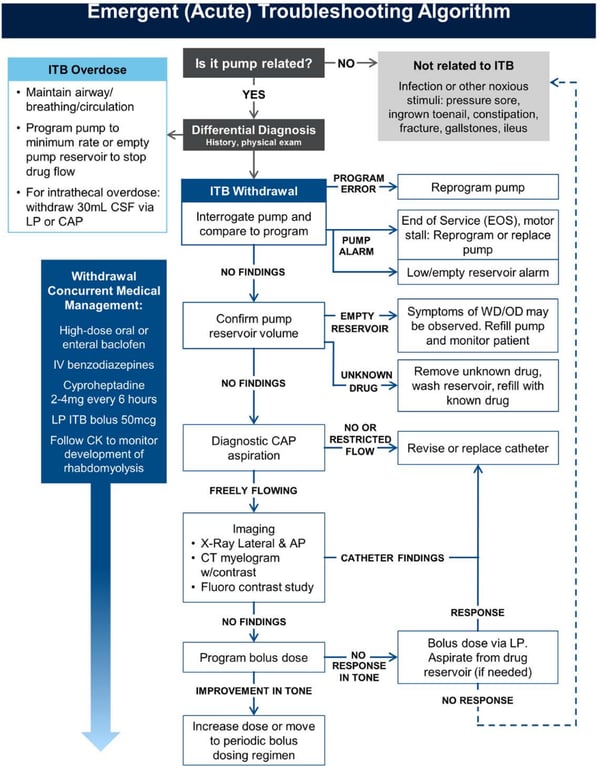
ITB emergencies
Work up
History
- Exposure to
- Whole body shock wave lithotripsy
- Potential for electronic damage to the device by the sound waves.
- Pressures from SCUBA diving and hyperbaric oxygen therapy
- Places the patient at risk for pump damage or temporary under-or overinfusion, particularly if exposure is repeated and the reservoir is not close to full volume
- High-dose radiation therapy
- There is also a case report of battery failure of an intrathecal delivery system following
- Medications
- Medications that result in central nervous system (CNS) depression can contribute to an appearance of excessive ITB exposure.
- Examples include benzodiazepines, non-benzodiazepine sleep aids, some tricyclic antidepressants, and barbiturates.
- Several agents have been reported to increase hypertonicity, including selective serotonin reuptake inhibitors (24), interferons, dextroamphetamine, and theophylline.
- The patient’s prior experience with intrathecal drug delivery should be obtained from the clinician actively managing the patient.
- Date of implant
- Last refill
- Recent dosing adjustment
- Baclofen concentration changes
- Pump/catheter model number. Direct communication with the managing physician is a crucial step in triage and helps avoid redundant procedures.
- It may be worth consideration to assess patients who demonstrate gradually increasing dosing requirements or unusual dose escalation.
- In general, the panel recommends re-evaluating any ITB patient on a daily dose of 1000 mcg or higher. The panel recognized that this dose is very high and may represent a somewhat arbitrary demarcation
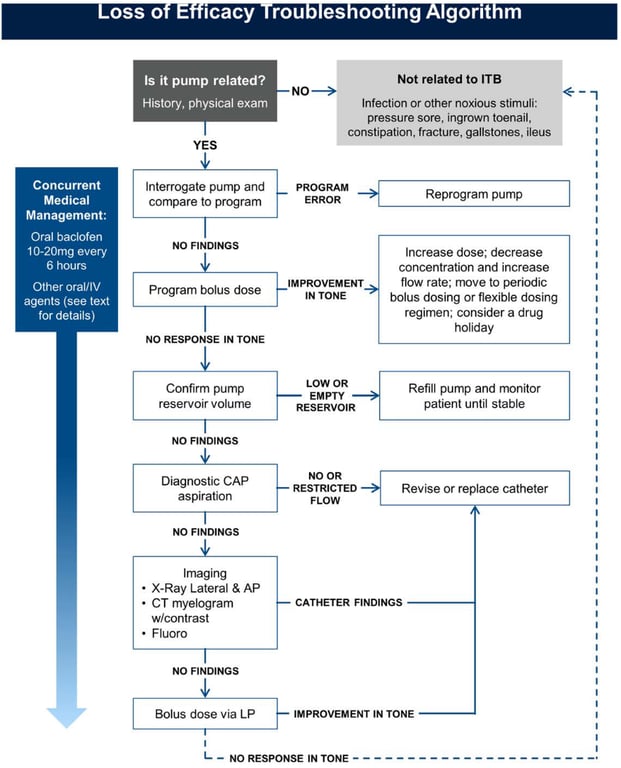
- Loss of Drug Effect
- Diagnosis of exclusion
- Pharmacologic tolerance
- Adaptive changes that occur within systems affected by the drug so that clinical response to the drug is reduced
- Tolerance has been implicated as a cause of acquired loss of response to ITB despite escalating ITB doses, often in the context of failure to demonstrate radiological evidence of an ITB system malfunction.
- Controversy exists regarding whether ITB response failure in such scenarios represents undiagnosed problems with drug administration through the pump and catheter system rather than actual pharmacodynamic tolerance in GABA-ergic target neurons of the spinal cord.
- Accordingly, a thorough analysis of any potential system malfunction should be undertaken before attributing dose escalation to tolerance
- Assuming the previously described steps have been undertaken without detection of system abnormalities, interventions for tolerance include decreasing the concentration of baclofen solution with a concomitant increase in flow rate, utilization of periodic bolus/flexible dosing, serial decrements in dosing followed by a re-titration, or a drug holiday
Physical examination
- Targeted neuromuscular examination (strength, range of motion, reflexes, clonus, spontaneous or elicited spasms)
- Many patients who utilize ITB therapy may also have preexisting sensory deficits, rendering some physical examination maneuvers unreliable.
- Eg:
- Acute abdominal pathology
- Long bone fractures in a patient with a spinal cord injury
- Vital signs
- Mental status examination.
- Look for noxious stimuli
- Suprapubic tenderness suggesting cystitis
- Flank pain suggesting kidney pathology
- The pump- and catheter-insertion sites should be inspected for signs of swelling, which could potentially indicate
- CSF leak
- Seroma
- Inadvertent subcutaneous drug injection (i.e., pocket fill).
- Communication with the managing physician is paramount in an effort to determine if there are any changes from baseline.
- Clinical signs and symptoms of baclofen toxicity and withdrawal.
System | Baclofen toxicity | Baclofen withdrawal |
General | Hypothermia, death | Pruritus, hyperthermia, multisystem organ failure, death |
Psychiatric | Hallucinations, agitation, mania, catatonia | Hallucinations, anxiety, paranoia, delusions |
Neurological | Hyporeflexia, tremor, confusion, impaired memory, lethargy, somnolence, seizures, encephalopathy, coma | Hyperreflexia, tremor, paresthesias, headache, altered mental status, delirium, seizures |
Cardiovascular | Conduction abnormalities, prolonged QTc interval, autonomic dysfunction: bradycardia, tachycardia, hypotension, hypertension | Acute reversible cardiomyopathy, cardiac arrest, autonomic dysfunction: bradycardia, tachycardia, hypotension, hypertension |
Respiratory | Respiratory failure | Respiratory failure |
Gastrointestinal | Nausea, vomiting | Nausea, vomiting, diarrhea |
Musculoskeletal | Hypotonia | Hypertonia, rhabdomyolysis |
Investigation
- Laboratory Assessment
- Assessment for infection and other noxious stimuli
- FBC
- U/E
- LFT
- Coagulation
- ITB withdrawal
- Multiorgan system failure
- In severe cases
- Presence of laboratory abnormalities does not “rule in” a withdrawal syndrome.
- Elevated creatinine phosphokinase (CK)
- The sensitivity and specificity of an elevation in CK has not been formally assessed.
- Rising serial CK levels to be associated with ongoing/worsening baclofen withdrawal
- Serum or CSF levels of baclofen are not particularly useful indicators.
- Pump interrogation
- The pump’s active dosing parameters should match the previously prescribed dosing.
- If a programming error is detected, reversion to the appropriate dosing level
- Presence of an audible pump alarm due to
- Low battery
- Low reservoir volume
- Refill should be undertaken.
- If there is any doubt as to the content of reservoir solution (such as drug concentration), then a new solution should be instilled.
- The low reservoir alarm in the Medtronic system is based on programmed settings and is not a measurement of actual volume.
- Therefore, premature emptying of the pump reservoir will not trigger the low reservoir alarm.
- There are rare reports of over infusion, as manifested by symptoms of overdosing and a lower-than-expected reservoir residual volume
- An increase in reservoir residual volume may suggest an abnormality of the pump rotor or a severely kinked catheter, resulting in underdosing or withdrawal.
- The presence of a permanent rotor stall, unexplained rotor stalls, overinfusion or low battery condition should prompt urgent replacement of the pump.
- Discovery of an unexpected “extra” residual volume in the reservoir
- Rotor stalling
- Due to high magnetic fields (such as during an MRI scan) exposure: will result in a temporary cessation of drug delivery.
- Rarely, there is a delayed restart of drug delivery with the associated underdosing /withdrawal phenomena.
- Detection of the rotor stall and restart can be assessed by inspection of the system’s internal logs.
- Rare cases of rotor stall in the absence of magnetic field exposure
- Diagnostic Bolus
- via
- Single ITB pump bolus dose comparable to or higher than the test dose (usually 50–100 mcg) can be programmed
- Reevaluated pt spasms over the next few hours.
- Bolus administration (single or multiple) via lumbar puncture.
- Troubleshooting
- Positive response to a LP bolus vs pump bolus is diagnostic of a system malfunction, and in the presence of a functioning pump requires immediate catheter replacement.
- Radiographically might see breakage or blockage
- Absence of radiographic findings suggests the possibility of microtears.
- Diagnostic Catheter
- Access Port Aspiration Catheter patency or malfunction may be suggested by the result of catheter access port aspiration (CAP)
- Diagnostic CAP aspiration may be performed after the pump is confirmed to be functioning through interrogation and pump reservoir volume verification.
- This procedure involves accessing a port that is in direct continuity with the catheter.
- If the distal end of the catheter lies within the subarachnoid space, CSF should be readily withdrawn through the catheter.
- Aspiration of only 2–3 mL is sufficient for determination of a normal aspiration since the volume of the catheter is typically less than 0.25 mL.
- Trouble shoot
- Failure to aspirate fluid strongly suggests catheter → Disruption OR Occlusion
- Need to revise catheter
- Difficult to aspirate → Partial catheter occlusion has occurred
- Further diagnostic workup with scintigraphy may be necessary.
- If the catheter cannot be aspirated, DO NOT perform a catheter contrast study due to the risks of overdose from drug in the catheter.
- Pump/Catheter Imaging
- X-ray
- Be aware: some catheters are radiolucent
- A flat AP plate of the abdomen,
- Lateral lumbar and thoracic spine series
- A contrast study
- To visualize the catheter and verify catheter tip location.
- Via
- CT
- The CT-myelogram also has the advantage of providing structural information relative to other organs that could be serving as noxious stimuli.
- Fluoroscopically
- Technique
- Once the CAP and catheter have been cleared of the drug solution and CSF obtained, contrast medium can be injected
- Contrast should not be injected if 2– 3 mL of CSF cannot be easily aspirated, since this can potentially expose the patient to an ITB overdose from infusion of drug remaining in the catheter
- A priming bolus must be programmed after a successful CAP aspiration to avoid subsequent underdose and potentially acute withdrawal.
- If real-time fluoroscopy is available, pump rotor function can be observed after programming a 90-degree pump rotor rotation.
- This confirms the rotor arm is moving and effectively excludes mechanical pump dysfunction
- Extravasation of contrast out of the catheter =
- Catheter breaks,
- Catheter tip loculations
- Catheter migration into the subdural or epidural spaces
- Radionuclide scintigraphy
- Indium 111 DTPA can be injected into the pump reservoir and used as a tracer to determine the patency of the infusion system.
- After injection, serial sequential scanning occurs every 24 hours for two to three days.
- Normal studies should demonstrate an intact catheter and full ventriculogram.
- This technique can detect evidence of catheter occlusion, pump malfunctions, and large leaks.
- Disadvantages
- Cost
- The need for two to three days to confirm the abnormality
- Limited anatomic resolution
- Potentially high false negative rate
- Need to do careful calculations to determine proper timing of imaging,
- Poor access by some centers to this technology.
- Indium 111 DTPA has not been tested or approved for delivery through intrathecal pumps
- MRI imaging
- Thoracic spine can demonstrate
- Spinal hemorrhage,
- Abscess,
- Granuloma at catheter tip.
- These have only been pathologically confirmed with intrathecal opiate therapy for chronic pain.
- While rare, granulomas have the potential to cause serious neurologic injury from spinal cord compression.
- MRI imaging of the catheter tip with gadolinium contrast is the diagnostic test of choice for granuloma detection.