Principles of radiobiology
- Radiation causes cellular damage and selects cancer cells which are less tolerant to damage than normal cells
- Irradiation of nucleated cells → double- strand break (DSB) in the DNA
- The two major DNA DSB repair pathways in mammalian cells are
- Non-homologous end- joining (NHEJ)
- NHEJ is active throughout the cell cycle
- NHEJ is error prone since DNA sequence can be lost between re- joined strand ends. → Repair by NHEJ may therefore be mutagenic and contribute to radiation- induced malignancy.
- Homologous recombination (HR).
- Active during S and G2 phases
- When a sister chromatid is present to act as a template for repair of the damaged strand,
- HR is generally error free
- Since a normal template is used to regenerate damaged DNA,
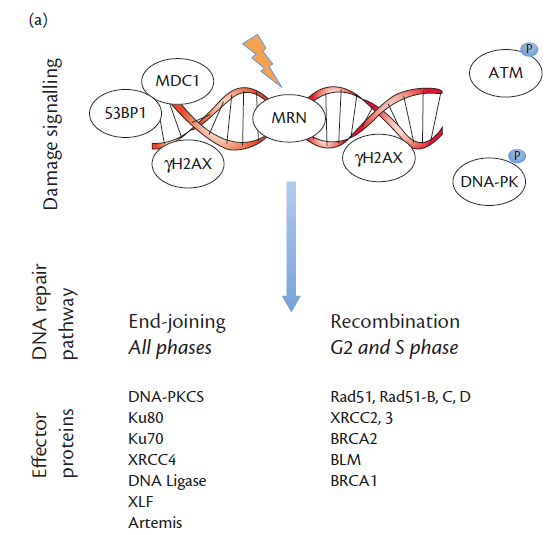
The signalling events and major effector proteins in each pathway are summarized:
- Augmenting radiation DNA damage
- Via targeting DNA repair pathways (aka Synthetic lethality)
- Eg:
- High grade glioma
- RT + Temozolomide in
- Is shown to improve outcome
- Due to addictive effects rather than TMZ causing damage in MGMT methylated tumours
- When Methylated MGMT cannot function to remove mthyl groups from the crosslinked DNA → better response to radiotherapy.
- Rivera 2010: The median time interval between resection and tumor progression of unmethylated tumors was also nearly half that of methylated tumors.
- Cellular survival vs radiation dosage
- Most cell have DNA repair capability
- Tumour cells have poorer DNA repair capability where as normal cells have better DNA repair ability → so if large total radiation doses that are given in small fractions they are better tolerated by normal cells
- This allows normal tissue, which is less able to tolerate large single doses, to repair and regenerate following treatment.
- A standard radiotherapy regime is given as daily doses (fractions) of around 2 Gy x5 days per week for 6 weeks = 60 Gy
- SRS that exclude normal tissue in treatment field can give larger doses per day or single large doses.
Physics of radiotherapy
- Modern radiotherapy makes optimal use of the physics of high energy (kilovoltage range) X- rays to deliver treatment.
- How radiation for RT is made
- An electron beam is accelerated to a metal target, producing secondary X- rays
- X-rays are filtered and collimated to produce a homogenous beam of a set
- Energy range
- Shape
- Beam energy
- Determines the depth in tissue where most of the beam energy and therefore cellular damage is deposited.
- For high energy beams, this is in the range of several centimetres.
- Reducing normal tissue exposure
- Multiple beams from different directions
- Where the beams cross highest dose (at tumour)
- Reducing dose to non- target tissue in the beam entry paths.
- Delivering treatment in a dynamic manner so that a smaller volume of normal tissue is exposed for any given tumour dose.
The 5 R of radiotherapy
Radiosensitivity
- Refers to the intrinsic radiosensitivity/radioresistance of certain normal and tumor cell types:
- Radiosensitive: hematological cells, epithelial stem cells, gametes; hematological and germ cell malignancy
- Paediatric
- Germinomas,
- Non-germinomatous germ cell tumors,
- Medulloblastoma,
- PNET,
- AT/RT,
- Choroid plexus carcinoma.
- Radioresistant: myocytes, neurons; melanoma, sarcoma
- Paediatric
- HGG
- Ependymoma
- Choroid plexus papilloma
- DNET
Repair
- Ionizing radiation can cause lethal, sublethal and potentially lethal damage to cells. If radiotherapy is fractionated, sublethal damage can be repaired before the next dose— and this repair occurs more effectively in normal tissue compared to malignant cells (e.g., TP53 mutation). If multiple fractions are to be given on the same day, the repair half-life of the normal tissue must be considered, e.g., 4 h repair half-life in spinal cord therefore separate fractions by 8 h
Redistribution
- When radiotherapy is given to a population of cells, they may be in different parts of the cell cycle. Cells in S-phase are typically radioresistant, whereas those in late G2 and M phase are relatively radiosensitive. A small dose of radiation delivered over a short time period will kill a lot of the sensitive cells and less of the resistant cells. Over time, the surviving cells will redistribute the proportion in each cell cycle phase. Surviving cells that now moved into a more radiosensitive phase can now be killed with a second fraction of radiotherapy
Reoxygenation
- Radiotherapy targets both normal and tumor cells by producing free radicals that damage DNA, but normal cells can repair this damage more effectively.
- Tumor cells can be more resistant to radiotherapy if they become hypoxic,
- Fractionating the treatment can reduce the likelihood of hypoxia during therapy and increase the chances of killing tumor cells.
Repopulation
- Post-radiotherapy, an increase in cell division is observed in both normal and malignant tissues, which influences the duration and timing of the treatment.
- Normal tissues show effects at 4 weeks, while some tumors may exhibit dangerous accelerated repopulation around 4-5 weeks, necessitating additional treatment fractions.
- Extending treatment beyond 4 weeks can reduce normal tissue reactions but also increases the risk of tumor repopulation.
Target
Target Volume Characteristics | Condition |
Target volume late responding as contains normal and abnormal tissue | AVM |
Target volume late responding but only in abnormal tissue and with marked radiobiologic effect | Benign tumor, e.g., meningioma, pituitary adenoma, vestibular schwannoma |
Target volume shows small, early effect on abnormal tissue but bigger, late effect on normal tissue (radiosurgery only indicated in some cases) | Low-grade glioma |
Target volume contains no normal tissue and abnormal tissue shows early radiobiologic effect | Metastasis |
Planning
Term | Definition |
Gross tumor volume (GTV) | Volume of macroscopic tumor that is visualized on imaging studies |
Clinical target volume (CTV) | Volume that should be treated to a high dose, typically incorporating both the GTV and volumes that are assumed to be at risk due to microscopic spread of the disease |
Planning target volume (PTV) | Volume that should be treated in order to ensure that the CTV is always treated, including considerations of systematic and random daily setup errors and intertreatment and intratreatment motion |
Organ at risk (OR) | Organ whose damage is especially dangerous and where a small amount of radiation damage would produce a severe clinical manifestation, e.g., spinal cord. Their radiation sensitivity influences treatment planning or prescribed radiation dose |
Planning organ at risk volume (PRV) | Margin added around the OR to account for uncertainties in planning and delivery |
Systematic target volume (STV) | Margin added to the CTV to account for systematic errors arising from treatment planning |
- PTV= CTV + STV
- CTV = GTV + volume of microscopic spread
Dosage
- GBM: Strupp protocol
- 2Gyx30=60Gy + TMZ
- LGG
- 54 Gy=30 fractions X 1.8 Gy
- Over a period of 6 weeks
- Brain Metastasis
- Standard
- 20Gy in 5 fractions
- 30 Gy in 10 fractions
- Prolonged regiment
- 40Gy = 2Gy in 20 sessions
- Brain stem
- <15Gy
- Max radiation dose for tumours near the optic nerve is <8Gy
- Lower cranial nerve can tolerate <25 Gy
Limitations and side effects of radiotherapy
- Despite optimization of complex radiotherapy delivery methods, non- tumour tissue can rarely be totally excluded from exposure to radiation during treatment.
- In the CNS, the relevant normal tissues are the skin and hair on the scalp, non- involved eloquent brain, the optic and auditory apparatuses, the pituitary gland, and the cerebral vasculature.
- All of these tissues show dose- dependent toxicity, expressed over a time course that depends on the tissue involved.
- A 5 year old age limit for Radiotherapy (theoratical risk): Grundy 2007
- Can resultant reduction in IQ and cognitive impairment, endocrinopathy, and risk of second malignancy
- Side effects are conventionally grouped according to the time at which they are expressed
- Early effects in weeks
- Common early toxicities include hair loss and skin erythema, within 3– 4 weeks of commencing treatment.
- Intermediate effects
- Manifesting as a somnolence syndrome
- Late effects
- Months to years
- Late toxicity mechanisms
- Are linked to damage in the CNS's endothelial and glial compartments.
- CNS cells are mostly post mitotic, with oligodendrocytes being the most radiation-sensitive.
- Demyelination can occur at relatively low radiation doses.
- Late toxicity models include stem-like cell loss in specific brain areas, potentially leading to cognitive deficits.
- Clinical data suggests stem cell populations are sensitive to very low radiation doses.
- Radiotherapy planning now tries to exclude stem-cell-rich areas from high-dose exposure.
- Hippocampal sparing approaches using IMRT techniques are deliverable but lack randomized data on neurocognitive outcomes.
- Drugs like memantine may act as neuroprotectants during radiotherapy, but studies show no significant benefit thus far.
- Includes
- Hormone failure
- Damage to visual and auditory pathways
- Cognitive deficit
- Can resultant reduction in IQ and cognitive impairment, endocrinopathy, and risk of second malignancy
- Due to reductions in hippocampal neurogenesis
- eg:
- Ependymoma: 5 year old age limit for Radiotherapy (theoratical risk): Grundy 2007
- Medulloblastoma: 3 year old age limit
- ATRT: 1.5 year old age limit
- Low grade tumour 40 year old age limit
- Cognitive harm from WBRT in mets (Generate hyperlink)
- Malignancy risk
- Incidence of radiation-induced malignancy
- Pediatric data suggests a 5-10 times higher malignancy risk from brain irradiation compared to non-irradiated groups, with younger age at exposure increasing the risk.
- Epidemiologic data, such as A-bomb survivor data, shows a dose-proportional malignancy risk, but converting whole-body doses to risk estimates for partial organ volume exposure is difficult.
- Techniques to reduce radiation to normal tissue
- Modern radiotherapy techniques, like IMRT, deliver more accurate doses to tumors and reduce high-dose exposure to surrounding tissue.
- However, techniques like IMRT may increase low-dose radiation exposure to normal tissue, and the long-term risk contribution of this is unknown.
- No studies have reported on second cancer risk after brain radiotherapy using IMRT or proton beam therapy.
- SMART (stroke-like migraine attacks after radiation therapy) syndrome
- Rare complication of brain radiotherapy with delayed onset of complex neurological impairment unrelated to tumor recurrence
- More frequently seen in men with male to female ratio of 2.2:1
- Interval between radiotherapy and the diagnosis of SMART ranges from 1 to 35 years
- Associated with the age of the patient when the radiotherapy was given
- Hallmark features of SMART on MRI are reversible, transient, unilateral cortical gadolinium enhancement as well as the correlative abnormal T2 and FLAIR (fluid attenuated inversion recovery) signal
- A long term complication of cranial radiotherapy, not systemic radiotherapy or chemotherapy