Summary
ㅤ | LINAC (e.g., CyberKnife) | Gamma Knife | Proton Beam Therapy |
Source | Linear accelerator shoots electrons at tungsten target | Cobalt-60 decay | Cyclotron |
Rays | X-rays | Gamma rays | Proton |
Head immobilization | Stereotactic frame or frameless (fiducials) | Stereotactic frame | Immobilization |
ㅤ | Machine moves around patient during treatment | Equipment stationary | Equipment stationary |
Multileaf collimator | In machine | In patient helmet | ㅤ |
Use | Whole body | Intracranial only | Whole body |
Type | Notes |
Radiotherapy | ㅤ |
Conformal radiotherapy (3DCRT) | This uses a device inside the radiotherapy machine to shape the radiotherapy beams to the target in three dimensions (height, width, and depth). The desired cross-sectional shape of the beam can be formed using blocks or a multileaf collimator. Beams can be fixed or intensity-modulated |
Whole brain radiotherapy (WBRT) | Non-conformal, two-dimensional radiotherapy (opposed lateral fixed beams) |
Intensity-modulated radiotherapy (IMRT) | Shapes the radiotherapy beams to allow different doses of radiotherapy to be given to different parts of the treatment area. This means lower doses of radiotherapy can be given to normal tissue, hence often used close to organs at risk |
TomoTherapy | Hybrid between CT scanner and IMRT—the radiation source for both radiotherapy and CT imaging can move completely around the patient in a helical arc. CT scans performed immediately before treatment. Highly conformal and precise, conformal avoidance of normal tissue but slower than VMAT. No comparison studies available currently |
Volumetric modulated arc radiotherapy | Type of IMRT using rotational (arc) delivery. The angle of the beam, the dose rate, and the leaf speed are all independently controlled, making this a very accurate form of treatment. Arc therapy treatments also take much less time to deliver than other radiotherapy techniques |
Image guided radiotherapy (IGRT) | Refers to any mode of radiotherapy where imaging of the tumor is performed during treatment to ensure treatment precision. Could be between several fractions, immediately prior to each dose (e.g., tomotherapy), or in real-time (e.g., CyberKnife® radiosurgery) |
Photon (X-ray) radiosurgery | ㅤ |
Gamma Knife | Use multileaf collimator in a helmet attached to a stereotactic head frame allowing multiple small beams to deliver high dose to small target small deep lesions |
Frame-based Linac | Uses stereotactic head frame to and multileaf collimator in the linac radiosurgery machine |
Frameless Linac (CyberKnife Robotic Radiosurgery) | Uses a moving couch and a small linear accelerator on a robotic arm to deliver multiple beams of radiation from different angles. It works best on small tumors with well-defined edges. Due to real-time image-guidance it can also adjust the delivery, for example to match the patient’s breathing motion |
Fractionated stereotactic radiotherapy | Combines the similar dose conformality, precise dose delivery, and steep dose falloff outside the target volume of stereotactic radiosurgery with the radiobiologic advantages of dose fractionation. Fractional safety targets larger tumor volumes intimate to critical structures such as the optic apparatus |
Particle (Hadron) radiosurgery | ㅤ |
Proton beam therapy | Protons deliver a dose of radiation in a much more confined way to the tumor tissue than photons (X-rays, gamma rays). After they enter the body, protons release most of their energy within the tumor region and, unlike photons, deliver only a minimal dose beyond the tumor boundaries. Therefore, especially for smaller tumor sizes, the dose of radiation may conform much tighter to the tumor and there may be less damage to healthy tissue. In particular its use in young children has been increasing, especially where conventional radiotherapy may be contraindicated due to its effects on CNS development |
Fast-neutron therapy | Theoretical advantage over photons in low-oxygen (hypoxic) conditions, but interest in the use of neutron therapy has waned because it has shown no advantages in terms of outcome over irradiation with other types of particles |
Carbon-ion therapy | Combines the dose-distribution advantages of protons with an increase in biologic effectiveness toward the end of the particle range |
Boron-neutron capture therapy | Clinical studies undergoing in recurrent malignant gliomas |
Conventional radiotherapy
- Brada 1992:
- Risk of developing a second brain tumour
- 10 years after RT was 1.3%
- 20 years after RT was 1.9%
Conformal external beam radiotherapy
- 3-4 beams static beams used to target a tumour
- Utilizes 3D diagnostic imaging, usually from a combination of MRI and computed tomography (CT).
- Importantly one of these data sets (usually CT) is derived when the patient is in the intended treatment position, so that the anatomy can be cross-reference to the treatment situation.
- The secondary imaging data set (usually MRI, or sometimes positron emission tomography (PET)) is then digitally superimposed on the CT image. The patient is immobilized in a personalized plastic mask for both CT imaging and for subsequent treatment, permitting accurate, conformal, and reproducible treatment for many tumour types.
- The treatment beams are then designed as described earlier to deliver a high dose to the tumour target and avoid as much normal tissue exposure as possible.
- Limitations
- When complex 3D targets are defined
- Eg Regions close to skull base.
- When radiosensitive organs at risk of radiation damage are located in concavities close to the tumour.
- Eg: Regions close to posterior orbit
- 3D- conformal treatment cannot then deliver high doses to the tumour while sparing normal tissue.
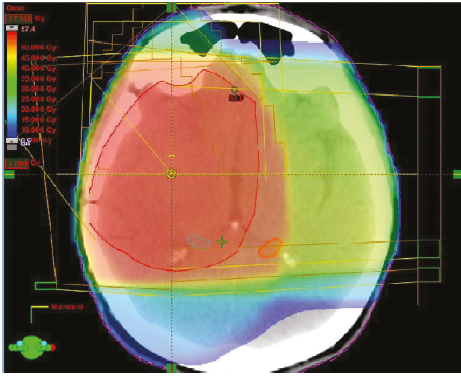
Intensity- modulated radiotherapy
- Delivers treatment using collimators to modulation the radiation beam.
- By using multileaf collimators that are moved within the beam path as it exits the machine head so that the beam is modulated in 3D before it reaches the patient.
- This permits much more complex shapes to be treated in a highly conformal way and specifically enables dose to be delivered around concave structures.
- The applicability of IMRT for treatment of brain cancers has been evaluated in clinical and dosimetric studies, which show that it can be used to target these tumours effectively
- Further developments in technology including
- Approaches in which IMRT is delivered more rapidly in an arc- mode have optimized IMRT delivery by reducing the beam ‘on time’ to reduce the period that the patient needs to be on the treatment couch.
- Pros
- Lowers the total body dose due to internal scatter which can be high when multiple beam directions are used.
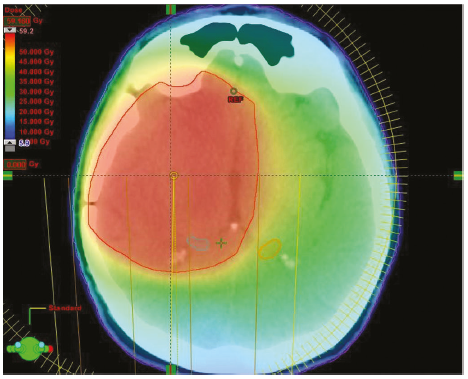
Stereotactic radiosurgery
- Radiosurgery is used to describe delivery of highly focused radiotherapy given as a single dose with the aim of producing local tumour ablation.
- Risk of secondary tumour after SRS: 0.04% at 15 years
- Types
- Gamma Knife
- Older technology
- Technique
- Delivered using cobalt- based technology in which multiple sources are targeted at a single point, producing a very high dose at the intersection of the radiation beams (isocentre) with a steep dose fall off beyond.
- In this approach patients are immobilized in a head- frame to ensure accurate delivery in 3D space.
- Cyberknife
- Uses image guidance to deliver radiosurgery through a robotic gantry without the need for an immobilization frame.
Cyberknife | Gamma knife |
Xrays | Gamma rays |
Whole body | Only above brain (supratentorial) |
- Used in
- AVM
- Cavernoma: controversial as outcome seem similar to placebo
- Trigeminal schwannoma
- Vestibular schwannoma
- Obsessive compulsive disorder
- Treatment targeted at anterior internal capsule bilaterally
- Meningioma:
- Risk of peritumoural oedema is increased
- High dose
- Treatment volume > 5cm3
- Brain tumour interface of >1cm2
- Presence of pre tx oedema
- Parasagittal location
- Pituitary adenoma
- Metastasis
- Planning
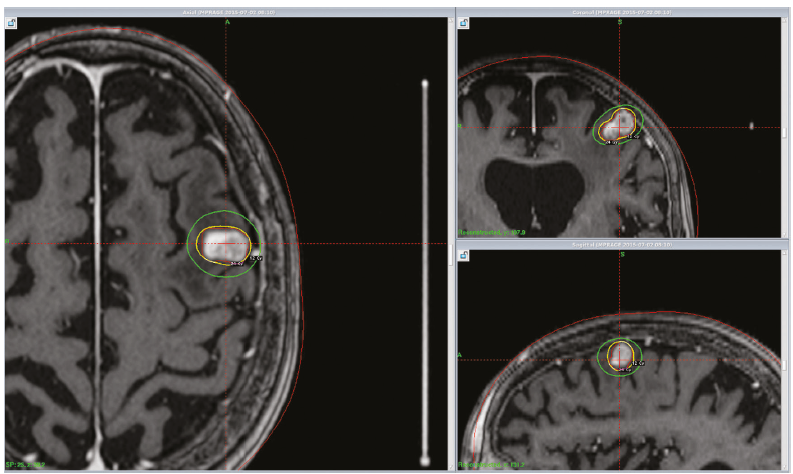
- CI
- Near optic nerve
- Guidelines for reducing the risk of normal-tissue toxicity
- The interaction of dose and volume irradiated has not been clearly defined for most structures at risk.
- Normal tissues at risk depend on the location of the target volume.
- Brain parenchyma (edema, necrosis),
- Brainstem (edema, necrosis, neuropathy)
- Cranial nerves (neuropathy),
- Hypothalamic-pituitary axis (hypopituitarism).
- Tissue toxicity
Structure | Single-Fraction Dose Constraint |
Brain lesion < 2 cm | 24 Gy (less than 20% risk of serious complication) |
Brain lesion 2-3 cm | 18 Gy (less than 20% risk of serious complication) |
Brain lesion 3-4 cm | 15 Gy (less than 20% risk of serious complication) |
Brainstem | 16 Gy—less than 5% cranial nerve deficit if less than 1/3 brainstem gets 16 Gy |
Pituitary | 15 Gy—no risk of hypopituitarism below this level |
Optic chiasm/nerve | 8 Gy—no risk of visual loss below this level |
Cranial nerves III, IV, VI | 30 Gy |
Trigeminal nerve | < 12.5-13 Gy |
Facial nerve | < 12.5-15 Gy |
Cochlea | < 3.7 Gy |
Spinal cord | 50 Gy |
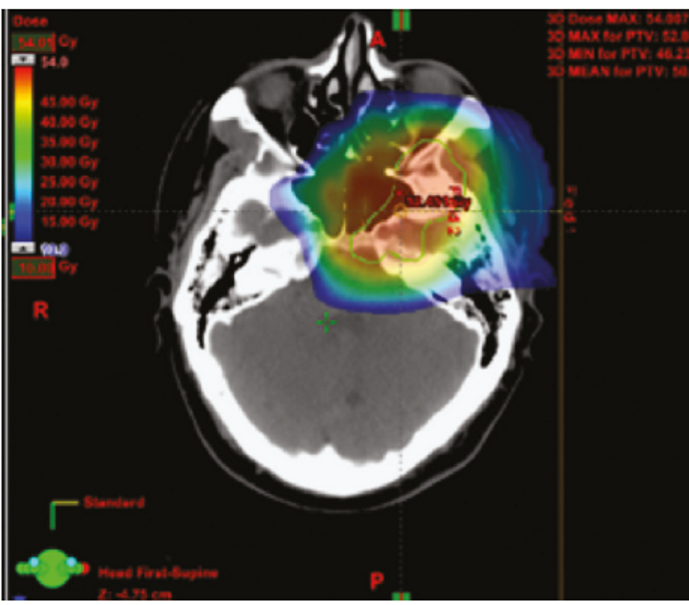
Proton beam therapy
- Utilizes charged proton particles
- The advantage of this approach comes from the specific dose profile of charged particles in tissue.
- There is a small range over which the proton energy is deposited called the Bragg peak, which is dependent on
- The energy of the beam
- The tissue that the beam traverses.
- Used in Chordomas
- Advantage
- Can be used to target a beam that delivers high-energy irradiation at a very specific point with practically no effect on deeper tissues.
- This is advantageous in situations
- In which normal tissue sits very close to a tumour target, or
- Where large volumes of radiosensitive tissue lie in the exit path of the beam.
- Proton beam radiotherapy can therefore be used in situations where conventional X- ray beam treatment would lead to an unacceptably high risk of normal tissue toxicity, for example
- Skull base
- Close to the optic pathway
- Close to the brain stem
- Close to the spinal cord.
- Paeds where developing normal tissue is particularly sensitive to radiation exposure so removing the exit beam dose reduces long- term toxicity risks significantly.
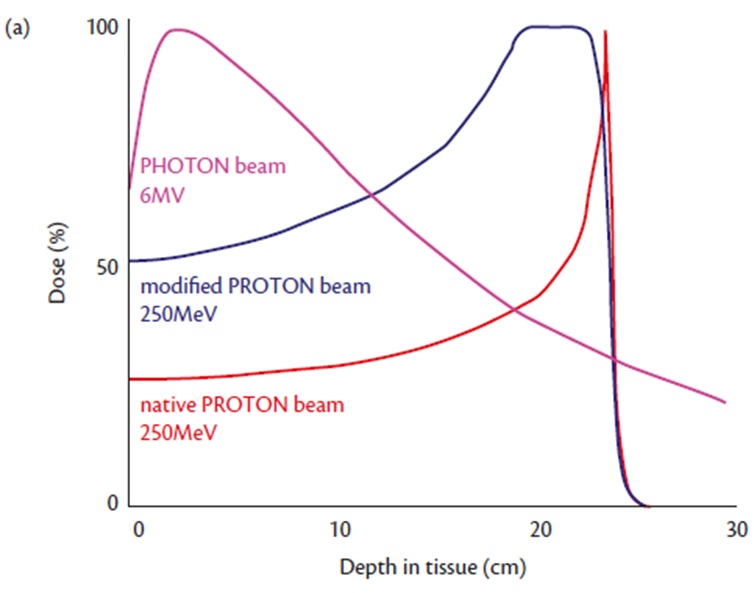
A representation of the dose deposition in tissue using proton beam (A)
A graphic representing the different dose deposition in tissue using a photon beam, with the maximal dose near the surface and significant dose beyond the tissue maximum (pink line).
In comparison a proton beam deposits dose over a narrow range of depth in tissue at the Bragg peak, with a steep dose fall off beyond (native proton beam, red line).
This can be modified to spread the Bragg peak over a wider range in tissue (modified proton beam, blue line) at the cost of some increased dose closer to the surface.
In comparison a proton beam deposits dose over a narrow range of depth in tissue at the Bragg peak, with a steep dose fall off beyond (native proton beam, red line).
This can be modified to spread the Bragg peak over a wider range in tissue (modified proton beam, blue line) at the cost of some increased dose closer to the surface.
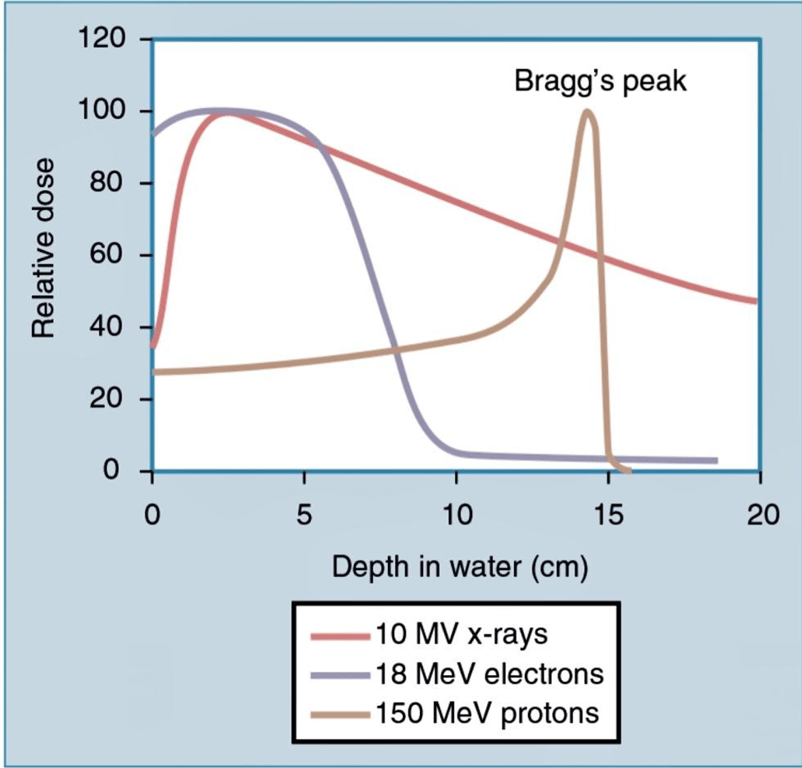
As a photon beam passes through material and is absorbed, the overall intensity of the beam is reduced.
In contrast, particles such as protons and ions travel a finite distance, which is termed the range.
They deposit a disproportionate amount of energy in the last few millimeters of their path.
- This large transfer of energy is known as the Bragg peak. The physical depth penetrated by the particles depends on tissue density and the beam’s energy.
Brachytherapy
- Treatment in which a radioactive source is placed directly in contact with the tumour or tumour bed target.
- Not really used in the brain
- Overall the response to brachytherapy in the brain has been disappointing with few studies demonstrating good local tumour control, but most demonstrating an increased risk of radionecrosis.
- More recently brachytherapy has been explored as an alternative to surgery for tumours at eloquent sites, however confirmation of the utility of this approach awaits assessment in randomized studies (Ruge et al., 2013).
Boron neutron capture therapy
- Boron neutron capture therapy is based on the principle of local enhancement of radiation dose.
- Achieved by delivering a boron containing compound (usually a boronated amino acid such as phenylalanine) to the tumour then delivering a local low- dose neutron beam to activate emission of alpha particles from boron.
- Tumour cells selectively uptake boron- amino acid compounds due to enhanced amino acid membrane transport systems, → selectively sensitize tumour cells to neutron irradiation.
- In principle this will also apply to scattered invading cells anywhere in the irradiated volume, making this an appealing approach.
- Challenges
- Effective delivery of boronated compounds in sufficient quantities for efficacy at the time of irradiation
- The specialist equipment required to produce and target the low energy neutron beam.
- Several groups worldwide are investigating this technique, but it is not yet widely available and has not been formally compared to conventional radiotherapy in clinical studies (Miyatake et al., 2009).