General
- Aka: BRG1-associated factors
- SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulators of chromatin (SMARC) alteration
Gene loci
- Chromosome 19p13.2.
Normal function
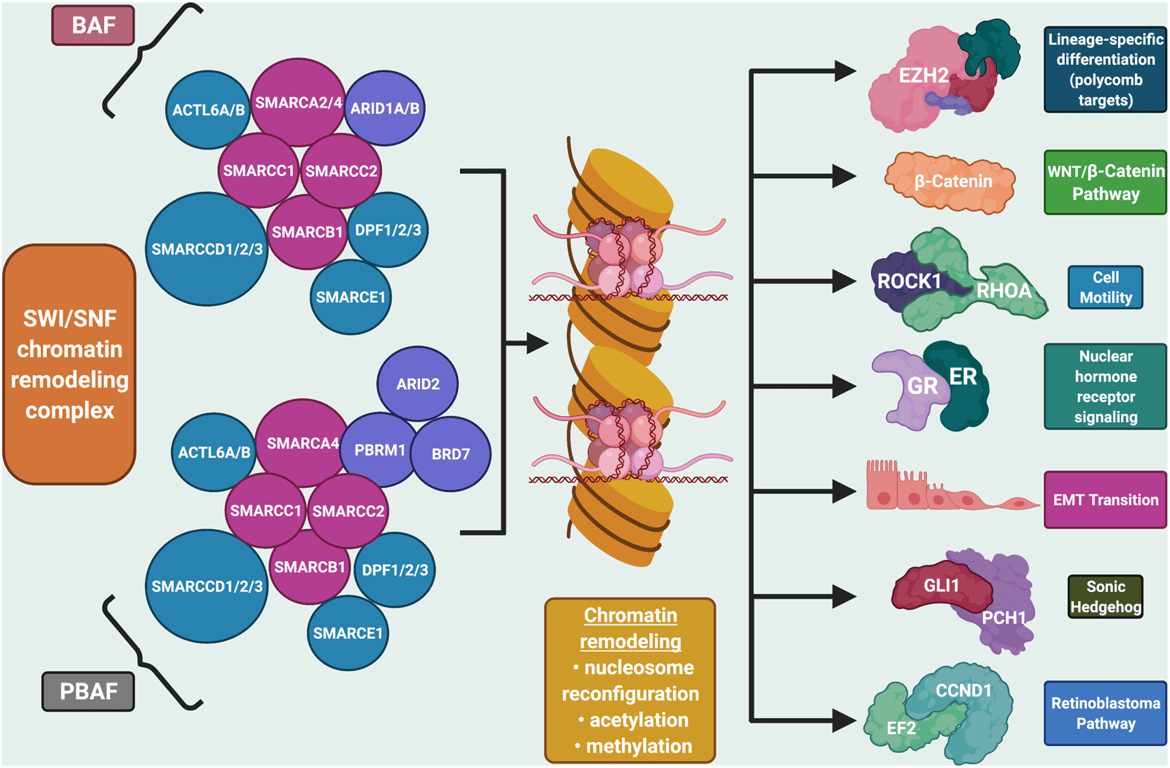
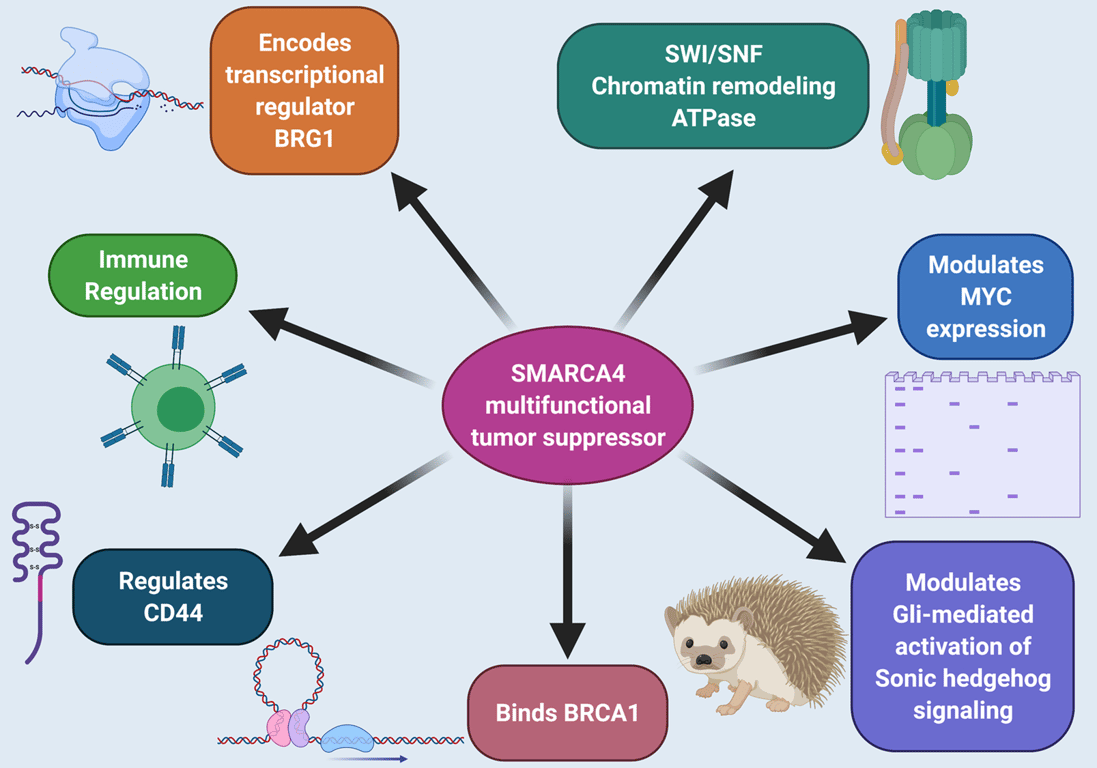
- Encodes BRG1, the ATPase subunit of the SWI/SNF (BAF/PBAF) chromatin‑remodeling complex, which uses ATP hydrolysis to reposition or eject nucleosomes and regulate transcription, DNA repair, and cell‑cycle control.
- Acts as a tumor suppressor by maintaining proper chromatin accessibility and transcriptional programs for differentiation and growth control.
Mutation effects
- Two types
- Class 1 mutations
- Truncating mutations, fusions, and homozygous deletion (loss of function usually associated with protein loss)
- Inactivating mutations, truncating variants, and deletions lead to loss of BRG1 protein, impaired ATPase activity, and defective chromatin remodeling, causing widespread transcriptional dysregulation.
- BRG1 (or SMARCA4) is the most frequently mutated chromatin remodelling ATPase in cancer
- Class 2 mutations
- Missense mutations (postulated to have dominant negative or gain of function effects, but some reports suggest loss of function, especially in lung cancer)
- Dominant-negative activity may be implicated in the context of a wild-type SMARCA4 allele (when present as heterozygous mutations) or dominant-negative activity with SMARCA2.
- Missense SMARCA4 mutations can also result in loss of accessibility and loss of chromatin remodeling activity.
- Germline loss‑of‑function variants cause dosage‑sensitive disruption of BAF complex function and developmental anomalies (Coffin–Siris spectrum, rhabdoid tumor predisposition).
Disease
- Germline pathogenic variants predispose to atypical teratoid/rhabdoid tumor, and other rhabdoid tumors (rhabdoid tumor predisposition syndrome type 2).
- Rhabdoid tumor predisposition syndrome (RTPS)
- Significantly increased risk of rhabdoid tumors before the age of 3
- Mutation
- SMARCA4 variants: ~5% to 15% (RTPS2)
- SMARCB1 variants: ~85%–95% (RTPS1)
- Rhabdoid tumors
- Location
- Can occur in almost any location
- Most commonly in the CNS; >50% in the cerebellum.
- Other common locations:
- Extracranial extrarenal malignant rhabdoid tumors (eMRT)
- Heart, bladder, liver, retroperitoneum, head and neck, paravertebral muscles, mediastinum, pelvis; rhabdoid tumor of the kidney (RTK); and
- SCCOHT (seen only in RTPS2, which is due to germline SMARCA4 alterations (and not in RTPS1 (with germline SMARCB1 alterations),
- These highly aggressive cancers are designated rhabdoid because their cells look like rhabdomyoblasts, which are cells that are normally seen in embryos and develop into muscles.
- 35% of pediatric malignant rhabdoid tumors are linked to germline SWI/SNF alterations
- SMARCA4 alterations are also seen in subsets of lung carcinomas, medulloblastoma and other cancers, reflecting its role as a broadly relevant tumor suppressor.