General
- SMARCB1: SWItch/sucrose nonfermentable (SWI/ SNF) related, matrix associated, actin dependent regulator of chromatin, subfamily B1
- Aka:
- INI1
- SNF5
- BAF47
Gene loci
- Chromosome 22q11.23.
Normal function
- ATP-dependent manner to alter chromatin structure
- SMACB1 and the SWI/SNF complex are actively involved in chromatin remodelling and are thought to have both transcription activation and repression roles
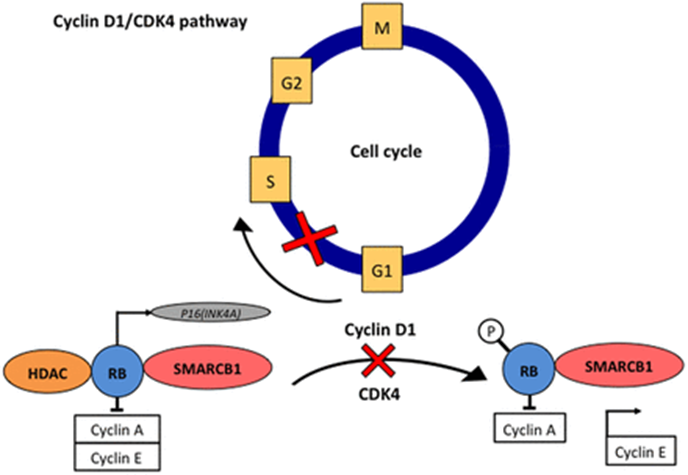
- SMARCB1 represses cyclin D1 → preventing G1 → S phase progression
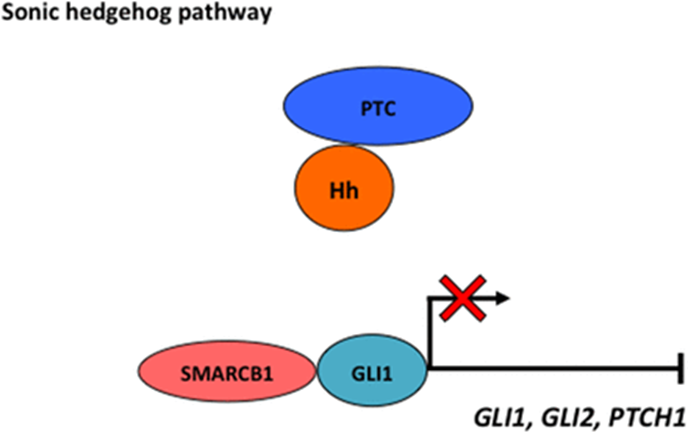
- SMARCB1 inhibits sonic Hedgehog pathway
- SHH pathway plays important roles in modulating patterning and differentiation during development
- SMARCB1 directly prevents transcription of glioma-associated oncogene homologue (GLI), thus resulting in reduced downstream SHH pathway target genes, including GL1, GL2 and protein patched homologue 1 (PTCH1)
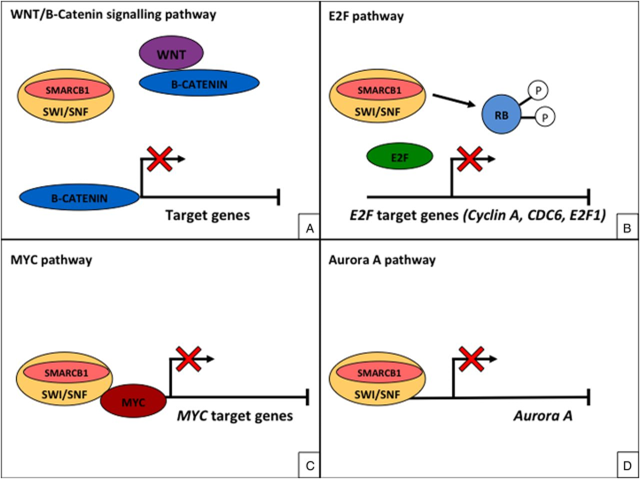
- SMARCB1 in regulation of WNT/B catenin pathway, E2F, aurora A and c-MYC
- Leading to transcriptional regulation of the cell cycle, development, proliferation and differentiation
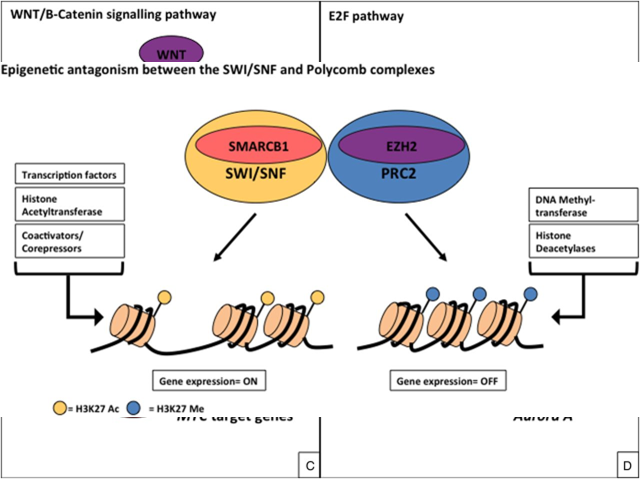
- SMARCB1 (SWI/SNF complex) epigenetic antagonism with polycomb complex
- During lineage-specific differentiation, the SWI/sucrose non-fermenting (SNF) complex (SMARCB1) interacts with transcription factors, histone acetyltransferases and transcriptional regulators to activate expression of target genes, which results in acetylation of lysine 27 of histone H3 (H3K27 Ac).
- Antagonising the actions of the SWI/SNF complex is the polycomb repressive complex 2 (PRC2), which contains enhancer of zeste H2 (EZH2).
- PRC2 interacts with DNA methyltransferases and histone deacetylases to silence gene expression, resulting in methylation of histone H3K27 Me.
Mutation effects
- Methods of mutation or loss of the SMARCB1
- The homozygous deletions of the SMARCB1 locus @22q11.2: 20-24% of cases
- Biallelic inactivation (deletions, truncating or splice mutations) leads to complete loss of SMARCB1 protein, destabilizes SWI/SNF complexes, and markedly impairs enhancer targeting, especially at differentiation‑related enhancers, causing widespread epigenetic dysregulation.
- SMARCB1 loss drives uncontrolled proliferation, alters cell identity programs, upregulates oncogenic pathways (e.g. MYC, MIR17HG), and creates specific therapeutic vulnerabilities such as dependence on CDK4/6 or BRD9.
- Other cases, one SMARCB1 allele is mutated and the second allele is lost by deletion or mitotic recombination.
Clinical association
- Genetic hallmark of AT/RT
- Most of the tumours have detectable deletions or mutations of SMARCB1:
- The other cases exhibit loss of SMARCB1 function due to reduced RNA or protein expression
- Virtually all atypical teratoid/rhabdoid tumors (ATRT) of the CNS and malignant rhabdoid tumors of kidney and soft tissue show complete SMARCB1 loss and represent prototypical SMARCB1‑deficient cancers.
- Germline SMARCB1 mutations underlie rhabdoid tumor predisposition syndrome type 1 and are also associated with schwannomatosis and other tumor‑predisposition phenotypes.
- Additional SMARCB1‑deficient entities include epithelioid sarcoma, renal medullary carcinoma, some sinonasal carcinomas, and other rare pediatric and adult cancers, generally characterised by aggressive behavior and poor prognosis.
Cancer type | Loss of SMARCB1 expression (%) | Genetic alteration in SMARCB1 |
100 | Biallelic inactivation (whole-gene deletions, large intragenic deletions/duplications, insertions, splice-site mutations and nonsense mutations) | |
Epithelioid sarcoma | 80–90 | Homozygous deletions |
Renal medullary carcinoma | 100 | Loss of heterozygosity at SMARCB1 locus |
Medullary carcinoma | 40 paediatric 10 adults | Unknown |
Malignant peripheral nerve sheath tumour | 50 | Unknown |
Extraskeletal myxoid chondrosarcoma | 17 | Truncating mutations in both alleles of SMARCB1; homozygous deletions or microdeletions |
Familial schwannomatosis | 45* | Non-truncating splice-site mutations and missense mutations in exon 1 |
Desmoplastic myxoid tumor of the pineal region | SMARCB1-mutant |
- Tumours with histology features of AT/RT and intact SMARCB1 protein expression; these may instead have mutation and inactivation of SMARCA4, another component of the SWI/SNF complex
- These tumours are associated with very young patient age and a poor prognosis