General
- Abnormal collection of blood vessels where arterial blood flows directly into draining veins without normal interposed capillary beds.
- No brain parenchyma within nidus
- AVM are not congenital (not present at birth)
Definition
- Aggregates of abnormal arteries and veins of variable diameters with direct connections through a nidus or fistula instead of a normal capillary bed.
Classification
Layers
- Pial
- Subcortical
- Paraventricular
- Combined
Lawton location (7 types of AVMs, which are collectively organised into 32 subtypes)
Temporal AVMs
- Lateral temporal
- Basal temporal
- Medial temporal
- Sylvian temporal
Parieto-Occipital AVMs
- Lateral parieto-occipital
- Medial parieto-occipital
- Paramedian parieto-occipital
- Basal occipital
Ventricular and Periventricular AVMs
- Callosal
- Ventricular body
- Atrial
- Temporal horn
Deep AVMs
- Pure sylvian
- Insular
- Basal ganglial
- Thalamic
Brainstem AVMs
- Anterior midbrain
- Posterior midbrain
- Anterior pontine
- Lateral pontine
- Anterior medullary
- Lateral medullary
Mixed AVM
- 6.5% of the reviewed cases were classified as Mixed AVMs.
Localisation
- AVM have no predilection for specific central nervous system locations and are distributed proportionately within the brain mass.
Numbers
- Prevalence
- 0.14%
- Adults 10 -100 per 100 000 (Morris et al., 2009).
- Incidence:
- 0.9– 1.5 per 100 000 head of population per year (Al- Shahi et al., 2003; Stapf et al., 2003)
Grading
Spetzler martin grading
Size | Small <3cm | 1 |
ㅤ | Medium 3-6cm | 2 |
ㅤ | Large >6cm | 3 |
Eloquence | Non eloquent | 0 |
ㅤ | Eloquent | 1 |
Venous drainage | Superficial only | 0 |
ㅤ | Deep | 1 |
- Surgical outcome as per 100 cases by Spetzler
SM Grade | No. of pt | No deficit | Minor deficit | Major deficit |
1 | 23 | 100% | ㅤ | 0% |
2 | 21 | 95% | 5% | 0% |
3 | 25 | 84% | 12% | 4% |
4 | 15 | 73% | 20% | 7% |
5 | 16 | 69% | 19% | 12% |
- Eloquent=Sensorimotor, language, visual cortex, hypothalamus, thalamus, internal capsule, brainstem, cerebellar peduncle, deep cerebellar nuclei
- Superficial drainage = all drainage are superficial
- Deep drainage = internal cerebral vein basil vein of Rosenthal or pre-central cerebellar vein
- AVM mature at age 18 and tend to be more compact
Interpretation
- Spetzler 2011
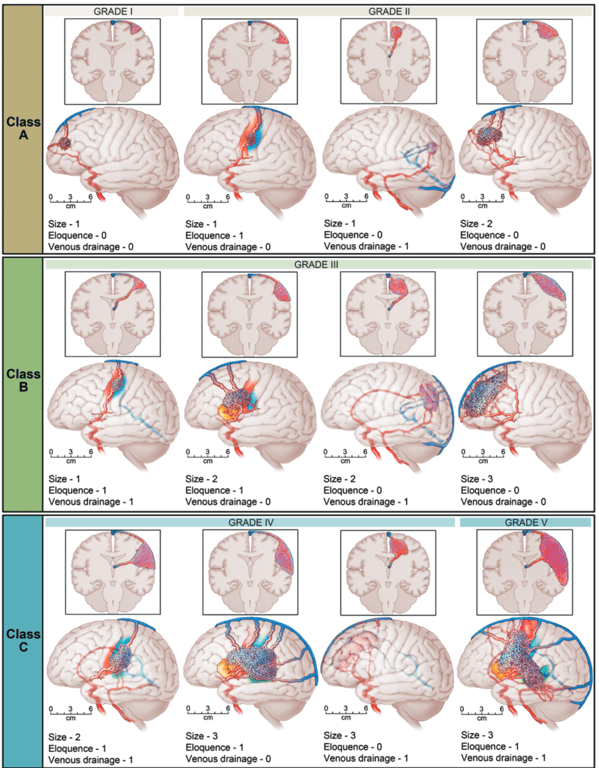
Class | SM score | Treatment |
A | I-2 | • Microsurgical resection is preferred treatment. • 8% chance on postoperative deficit |
B | 3 | • Multimodality treatment •18% chance on postoperative deficit |
C | 4-5 | • No treatment, with exception of recurrent hemorrhages, progressive neurological deficits, steal-related symptoms, and AVM-related aneurysms. • 32% chance on postoperative deficit |
Lawton and Young supplementary score
- = Spetzler martin grade + Lawton and Young supplementary score
- Formed because the grade 3 or group B is a mixed bag, some are easier to tx and some are harder
- Limitations
- Results of high volume surgeons- generalizability?
- Lack of externally validated outcomes
- Subjectivity: Diffuse v compact
- Useful framework for risk-assessment but doesn't replace judgment.
- Compact AVM has no brain matter in between nidus and diffuse if there is.
ㅤ | Characteristic | Score | |
A | Age | 0-20 | 1 |
ㅤ | ㅤ | 20-40 | 2 |
ㅤ | ㅤ | 40+ | 3 |
B | Bleeding | No | 1 |
ㅤ | ㅤ | Yes | 0 |
C | Compactness | Compact | 0 |
ㅤ | ㅤ | Diffuse | 1 |
- Interpretation
- Eg how the LY is a better and a more conservative risk calculator vs martin ponce
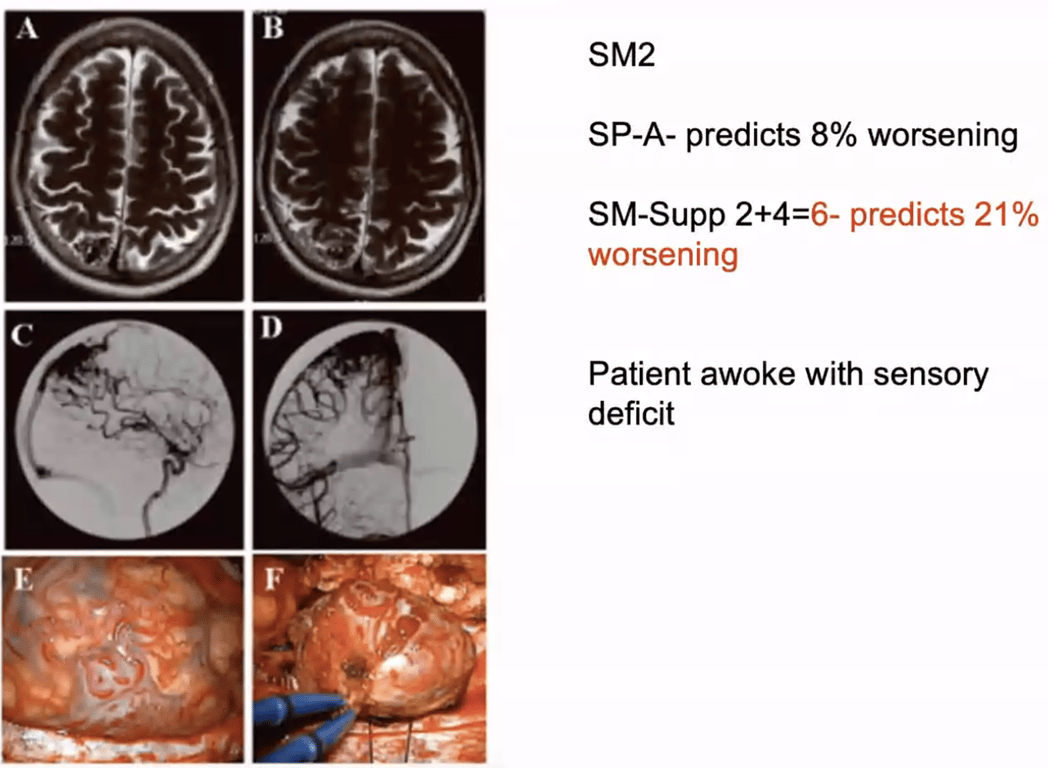
Pollock- Flickinger score
- Calculates the likelihood of obliteration without deficit from focused irradiation
- AVM score = (0.1)(AVM volume in cm3) + (0.02)(patient age in years) + (0.3)(location of lesion: frontal or temporal) = 0;
- Location of lesion
- Parietal, occipital, intraventricular, corpus callosum, cerebellar = 1
- Basal ganglia, thalamic, or brainstem = 2
- Outcome
- Chance (in %) of excellent outcome (with 95% CI)
- Chance (in %) of modified Rankin Scale decline (with 95% CI)
AVM score ≤1.00 | 89 (79-94) |
AVM score 1.01 - 1.50 | 70 (59-79) |
AVM score 1.51 - 2.00 | 64 (51-75) |
AVM score >2.00 | 46 (33-60) |
AVM score ≤1.00: | 0 (0-8) |
AVM score 1.01 - 1.50: | 13 (7-22) |
AVM score 1.51 - 2.00: | 20 (12-32) |
AVM score >2.00: | 36 (24-50) |
Comparison vs aneurysm
Description | AVM | Aneurysm |
Ratio | 1 | 6 |
Age of Dx | 33 | 43 |
Haemorrhage presentation | 50% | 92% |
Aetiology
- Congenital origin
- Bonnet- Dechaume Blanc syndrome
- Wyburn- Mason syndrome
- Hereditary haemorrhagic telangiectasia (HHT)
- Autosomal dominant capillary malformation
- Presents with skin capillary malformations
- AVM of the brain, limb, or face (no intra- abdominal or intrathoracic organ involvement) has been described with a RASA1 mutation
- Secondary
Pathophysiology
graph TD subgraph A ["Sporadic causes"] subgraph A1 ["Multiple gene involvement"] A11["Upregulation/downregulation<br>of multiple homeobox genes<br>(HoxD4 HoxB3)"] A12["Involve<br>with angiogenesis"] A11 --> A12 end A2["Somatic Kras mutations<br>(85% of AVM)"] end A --> C subgraph B ["Syndromic causes"] B1["Cerebrofacial arteriovenous<br>metameric syndrome (CAMS)"] B2["Hereditary haemorrhagic<br>telangiectasia (HHT)"] end B --> C subgraph C ["Models of AV shunt formation"] C1["Notch4 induced arteriovenous<br>shunt development from capillaries<br>(NOTCH3 gene In CADASIL)"] C2["Dilation and a primary<br>disorder of venules"] C3["Failure of regression of<br>primitive arteriovenous connections<br>during development"] end C --> D1 style C text-align:left D1["Formation of a nidus (conglomeration<br>of numerous AV shunts) that shunts bloods"] D1 --> E1 D1 --> D2 D2["High output Cardiac failure<br>(<1%)with pulmonary hypertension<br>can occur in neonates and infants"] E1["Due to lack of capillary bed"] E1 --> F1 F1["High pressure arterial bloods<br>enters venous system with low<br>resistance causes high flow of blood"] F1 --> G subgraph G ["Remodeling process"] subgraph G1 ["One"] G11["The high venous pressure and<br>high flow remodels the vein"] G12["dilate and walls to thin"] G11 --> G12 end subgraph G2 ["Two"] G21["The lower arterial pressure<br>remodels the artery"] --> G22["dilate"] end G3["This is why it is not congenitally<br>abnormal it requires time to form)"] G4["All these changes are mediated<br>through upregulation of eNOS and<br>down-regulation of endothelin along<br>with the remodelling vasculogenesis<br>factors (e.g. vascular endothelial<br>growth factor, VEGF)."] G5["There is usually no sharply<br>identifiable point where AVM can<br>be said to begin or end, rather<br>there is a transition zone reflecting<br>the pathological response to the<br>physiological perturbations."] end style G text-align:left G --> H1 G --> H2 G --> H3 G --> H4 G --> H5 G --> H6 H1["High flow vascular in arteries"] H1 --> H1_1 H1_1["Degeneration of remodelled<br>vessel wall"] H1_1 --> H1_2 subgraph H1_2 ["Aneurysm: (7% of AVM)"] H1_2a["Extranidal<br>Arterial aneurysms: located<br>on the wall of feeding arteries"] H1_2b["Unrelated aneurysm: arise<br>from vessels that are not<br>AVM feeders"] H1_2c["Flow related aneurysm: arise<br>from vessels that play a role in the<br>perfusion of the nidus and<br>(hemodynamically related to the AVM)"] H1_2d["Both can be either<br>Prenidal: proximal to the AVM nidus<br>Postnidal: distal to the AVM nidus"] H1_2e["Venous varices: located on<br>the wall of draining veins"] H1_2f["Intranidal:<br>Located within the boundaries of the nidus<br>Angiographically opacified before substantial<br>venous filling has occurred 75% in major<br>feeding arteries (inc. flow)"] end style H1_2 text-align:left H2["Haemorrhage (50%)"] H3["High flow in a low resistant<br>system will cause blood to be<br>stolen through the AVM"] H4["pressure is elevated within<br>the venous sinuses"] H5["If CPA AVM"] H6["Hb extravasation"] style A1 text-align:left style A2 text-align:left style B1 text-align:left style B2 text-align:left style C1 text-align:left style C2 text-align:left style C3 text-align:left style G1 text-align:left style G2 text-align:left style G3 text-align:left style G4 text-align:left style G5 text-align:left
Histopathology
Macroscopic
- Arteriovenous malformations show dilated surface draining veins and feeding arteries with a deep nidus.
Microscopic
- Arteriovenous malformations consist of variably sized abnormal arteries and veins with direct fistulous connections and intervening CNS tissue showing gliosis.
Natural history
General
- Probably a registry data is better than a RCT in finding the best way to treat AVM due to its rarity
- Patel et al., 2001: rarely spontaneously resolve
- 25% of patients that do not experience a haemorrhage will have a decline in function within a 10- year period
- Likely to be confined to larger AVM and be due to seizure or progressive neurological deficits
Bleeding risk
- Un-ruptured bAVM ICH risk is 1% per year (n=2, 525 IPDMA)
- CI = confidence interval; COL = Columbia; KPNC = Kaiser Permanente of Northern California; SIVMS = Scottish Intracranial Vascular Malformation Study; UCSF = University of California, San Francisco.
Cohort | Overall events | Overall rate | Overall 95% CI | Hemorrhagic events | Hemorrhagic rate | Hemorrhagic 95% CI | Non‑hemorrhagic events | Non‑hemorrhagic rate | Non‑hemorrhagic 95% CI |
All | 141 | 2.32 | 1.97–2.74 | 85 | 4.80 | 3.88–5.94 | 54 | 1.30 | 1.00–1.69 |
UCSF | 28 | 2.33 | 1.61–3.37 | 14 | 4.88 | 2.89–8.24 | 14 | 1.53 | 0.91–2.58 |
COL | 46 | 3.50 | 2.62–4.67 | 35 | 8.12 | 5.83–11.31 | 11 | 1.24 | 0.69–2.25 |
SIVMS | 14 | 2.37 | 1.40–4.00 | 9 | 5.54 | 2.88–10.85 | 5 | 1.17 | 0.43–2.80 |
KPNC | 53 | 1.79 | 1.37–2.34 | 27 | 3.04 | 2.06–4.43 | 26 | 1.25 | 0.85–1.84 |
Ave risk of haemorrhage is 3%/year (aneurysm is 3% if untreated and 1% of treated)
- Annual average haemorrhage rates for various AVM subgroup Stapf et al 2006
- Memory
- If prior haemorrhage: x5
- If Deep venous drainage: x3
- If deep nidus location: x3
Venous drainage | No prior haemorrhage | Prior haemorrhage | Nidus location |
No deep venous drainage | 0.9% | 4.5% | Not deep |
No deep venous drainage | 3.1% | 14.8% | Deep |
Deep venous drainage | 8.0% | 34.4% | Deep |
Deep venous drainage | 2.4% | 11.4% | Not deep |
The risk of bleed is not a constant number and it varies over the number years
- Probably need to tx young patients more
Bleeding risk in SM Grade 4/5 Sattari et al 2024
Category | Annual Risk of Hemorrhage (Natural History) | Risk of haemorrhage Post-Surgery | Risk of haemorrhage Post-SRS | Risk of haemorrhage Post-Embolization |
Cortical AVM | 2.68% | 0.74% | 5.35% | 16.96% |
Deep-Seated High-Grade AVM | 8.37% | 5.25% | 3.11% | 22.33% |
Annual and life time risk of haemorrhage
- Risk of bleeding (at least once) = 1 - (annual risk of not bleeding)^expected years of remaining life
- Assumption: constant risk of rebleeding after initial bleed
- No change in risk during lifetime (which is actually false)
- No difference in various location of AVM
Risk of haemorrhage increased with
(Koester et al 2023)
- Presence of aneurysm (OR = 1.45 [1.19, 1.77], p < 0.001
- Deep location (OR = 3.08 [2.56, 3.70], p < 0.001),
- Infratentorial location (OR = 2.79 [2.08, 3.75], p < 0.001)
- Exclusive deep venous drainage (OR = 2.50 [1.73, 3.61], p < 0.001) - into the Galenic system
- Single venous drainage (OR = 2.97 [1.93, 4.56], p < 0.001),
- Nidus size less than 3 cm (OR = 2.54 [1.41, 4.57], p = 0.002).
- Previous haemorrhage
Risk of (Morgan 2017)
- Neurological deficit or death after haemorrhage = 42%
- Death alone 9%
Clinical presentation with haemorrhage occurs in approximately 50%
- Untreated brain arteriovenous malformation | Neurology
- Multicenter AVM research study (MARS) - age and previous haemorrhage predict bleeding
- See Kim et al 2014
- 30% increase in risk of haemorrhage for each 10-year increase in age
Epilepsy
- Josephson 2011
- 5-year risk of first time seizure in AVM patients
- If patient had ICH/FND: 23%
- If patient had incidental AVMs: 8%
- SIVMS: No difference in seizure outcome between Conservative vs Invasive treatment
- Josephson 2015: Risk ratio intervention vs conservative (AED) = 0.99 (not much difference)
- Not enough evidence to prove that surgery can reduce seizure risk
- Rajeev 2022
- 19% of seizure naive patient develop epilepsy after surgery
- 1 year cumulative risk of 9%
- Higher risk of seizure when
- Temporal lobe AVM
- History of haemorrhage
Evaluation
CT
- Non-ruptured AVM
- Slightly hyperdense mass with a sharp border with the surrounding normal brain
- Calcification
- Ruptured AVM
- AVM may be obscured by the haematoma
MRI
- Unruptured
- Flow void on T1/T2 within AVM
- Feeding arteries
- Draining veins
- Significant oedema around lesion may indicate a tumour that has bled rather than AVM
- Gradient echo sequences help demonstrate surrounding hemosiderin which suggest a previous significant haemorrhage
- A complete ring of low density (due to hemosiderin) surrounding lesion suggest AVM over neoplasm
Angiography
- Allows for identification
- Draining vein
- Arterial supply
- Nidus
- Draining veins are in the same phase as arteries
- Angioarchitectural features such as diffuseness
- Presence of aneurysms
- Venous stenosis
Differential
- Proliferative angiopathy
- A response to infarct or ICH
- Particularly in the young
- Should be considered in diffuse vascular lesions that may resemble AVM but lack the very early venous drainage pattern of AVM.
- Do not share the same propensity to haemorrhage
Treatments
Difficult decision
Risk vs benefit
- Natural history
- Death 9%
- Disability 20%
- Intervention
- Death 0-5%
- Disability 1-24%
Conservative
- Conservative treatment may be appropriate in large lesions where therapeutic risk exceeds projected natural
history
- Observation
- Clinical signs (seizure/neurological deficits)
- Radiologically
- Evidence
- ARUBA study: Poor study
- Conservative medical management is better than intervention
- Conservative management resulted in a 10% risk of stroke or death and a 15% risk of disability over 33 months.
- Scottish intracranial vascular malformation study
- Conservative management had better clinical (death/handicap/haemorrhage) outcome
Intervention
General
Partial treatment is worse than natural history. IF YOU ARE NOT CONFIDENT TO COMPLETELY OBLITERATE IT DO NOT TOUCH IT WITH ANY MODALITY OF TREATMENT
- Haemorrhage risk before treatment
- n=61
- 42/61 had haemorrhages before treatment
- 22 haemorrhages under fu before treatment (3.49 years follow-up)
- 14/42 with previous haemorrhage
- 10.4% p.a. (95% CI, 2.2-15.4%)
- Haemorrhage risk after treatment
- 14 haemorrhages after treatment
- 6.1% p.a. (95% CI, 2.5-13.2%)
- 18/61 complete obliteration (and no haemorrhages)
- Hence do not debulk an AVM
- Hence you cannot palliatively treatment and AVM
Bleeding risk in SM Grade 4/5 Sattari et al 2024
Category | Annual Risk of Hemorrhage (Natural History) | Risk of haemorrhage Post-Surgery | Risk of haemorrhage Post-SRS | Risk of haemorrhage Post-Embolization |
Cortical AVM | 2.68% | 0.74% | 5.35% | 16.96% |
Deep-Seated High-Grade AVM | 8.37% | 5.25% | 3.11% | 22.33% |
Goal
- Obliterate the AV shunt completely
Indications for any treatment
- Bleeding
- Seizures
- Fear
By grade
- Spetzler-Martin Grade I and Il (Class A) AVMs
- Can be managed with microsurgical resection alone, achieving good outcomes in 96% and 90% of patients, respectively
- Class B or SpetzIer-Martin Grade Ill AVMs
- Case-by-case evaluation with special consideration for multimodal therapy.
- A conservative approach may be warranted in patients with these AVMs when they present without a history of rupture.
- Surgery if favourable anatomical location and no deep feeders
- Class C or Spetzler- Martin Grade IV and V AVMs,
- Conservative
- Intervention being considered only with progressive neurological decline and/or repetitive haemorrhage.
Evidence
- ARUBA study RCT
- Surgery had the highest cure rate
- 100% when combined with endovascular treatment
- Comparable morbidity
- Cons
- Small sample size
- What did we learn from ARUBA, SIVMS and MARS?
- Treatment of AVMs is associated with significant upfront risks.
- How is risk stratified?
- Institutional volume vs. outcome?
- We fail to obliterate AVMs frequently- why?
- Wrong choice of therapy?
- Therapy not well executed?
- Therapy not efficacious?
- More/better clinical trials are required to test established first-line therapies in comparable lesions? e.g:
- SMI/2 treated surgically vs. conservative treatment?
- SM3 AVM multimodality treatment vs. conservative treatment?
- Are RCTs really how to best address these questions?
Uncontroversial Statements to make in An Exam by Mr Walsh
- Surgical extirpation is generally preferred in cases where there has been recent haemorrhage- if feasible as
well as reasonably safe (prompt reduction in rebleeding - LYSScore - 0 for bleed
- Surgery in most cases can be a planned procedure
- 1st: decompress clot
- 2nd come back for AVM
- SRS is a reasonable alterative for small volume lesions that have haemorrhaged but without other
reasonably safe treatment alternatives (Balance the natural history of the ruptured AVM against projected
morbidity, efficacy and lead-time to occlusion)
- INR may be curative for favourable architectures
- Role of "palliative" embolisation unclear- one retrospective study suggesting slight improvement in
rebleeding with partial treatment, many others which do not support that conclusion - Targeted endovascular treatment of arterial bleeding points alone may reduce short-term risk of rebleeding
in high-grade lesions
Surgery
Options
- Haematoma evacuation + elective AVM treatment
- Is it safe YES: Beecher 2018 Delay treatment up to 4 weeks post <1% risk to patients
- Elective AVM treatment
- Definition of a favourable outcomes
- Obliteration of AVM + absence of new permanent neurological deficit in first 6 wks of surgery+ MRS>1 at 12 months post OP
- Timing
- Ruptured AVM
- Acute
- Considered for a patient when a rapidly declining neurological status is attributed to a ruptured AVM.
- Options
- Debulking of haematoma
- Treating AVM + debulking of haematoma
- Delayed
- General, a variable “rest period” (1–6 weeks) between the haemorrhage and the conclusive treatment.
- Pros
- Allow treatment planning for both radiosurgery and adjunctive embolization.
- Allow hematoma resorption → better radiological demonstration of the AVM.
- The treatment of choice for AVMs.
- When surgical risk is unacceptably high, alternative procedures may be an option
- Before surgery give 20mg PO QDS propranolol for 3 days to minimise post op normal perfusion pressure breakthrough → prevent post op bleed and oedema
- Post op keep MAP 70-80mmHg
- Techniques
- Pros
- Eliminates risk of bleeding almost immediately.
- Seizure control improves
- Best treatment for managing seizures
- Scottish intracranial vascular malformation study
- Medication first then surgery for seizure control
- Cons
- Invasive
- Risk of surgery
- Not suitable for deep/eloquent AVM
- Cost (high initial cost of treatment may be offset by effectiveness or may be increased by complications)
- Delayed post op deterioration
- Normal perfusion pressure break through and Occlusive hyperaemia:
- Arteriolar
- Since the AVM has low resistance to flow it causes reduce flow to normal high resistant arterioles in the periphery of the AVM → to compensate for this, the arterioles dilate to dec. resistance → chronic dilation causes arterioles to loose autoregulation
- When AVM removed, the low resistant, high flow steal is gone → but autoregulation is not present so the remaining good arterioles cannot change in shape to modulate the increased flow of blood to the dilated arteries → increase local blood pressure → cerebral oedema and haemorrhage
- Venous: post op the flow of blood in venous sinuses has dramatically reduce → predisposition to thrombosis which then causes back pressure to cause oedema and haemorrhage
- Rebleed from a retained nidus of AVM
- Seizures
- Commonest cause of post op bleeding in AVM is due to uncomplete removal of nidus
- Evidence
- Against surgery
- ARUBA study: RCT
- Scottish intracranial vascular malformation study
- For surgery
- Morgan 2017
- Risk of future AVM haemorrhage
- 8-Year Risk for Unfavourable Outcomes in AVM Patients
Condition | Time (Years) | Risk of Haemorrhage (%) | Annualized Risk (%) |
Without treatment (no haemorrhage) | 10 | 16% | 1.8% for unruptured AVMs |
ㅤ | 20 | 29% | ㅤ |
Without treatment (with haemorrhage) | 10 | 35% | 4.7% for 8 years for AVMs with haemorrhage followed by unruptured AVM rate |
ㅤ | 20 | 45% | ㅤ |
Condition | Size (cm) | Risk of Unfavourable Outcome (%) |
No Deep vein drainage (DVD) or eloquent location | 1 | 1% |
ㅤ | 6 | 9% |
Either DVD or eloquent location (not both) | 1 | 4% |
ㅤ | 6 | 35% |
Both DVD and eloquent location | 1 | 12% |
ㅤ | 3 | 38% |
Endovascular techniques
Options
- Embolization
Not to be used as Curative but supplement SRS or surgery
- As embolization itself has increased complication rates
Technique
- Occlude the arterial compartment of the AVM first to avoid bleeding complications associated with early occlusion of venous drainage.
- In contrast to Spinal AVF which embolizes the vein first
Pros
- Facilitates surgery
- By reducing blood loss in large AVM
- However, the overall management morbidity, mortality and success may not be improved with Embo prior to surgery approach as compared with surgery alone (Morgan et al., 2013; Bervini et al., 2014; Korja et al., 2014)
- Securing associated aneurysm or deep arterial feeders
- ? Fascilitates SRS
- The subsequent radiosurgery is less likely to obliterate residual volume AVM than equivalent volumes of AVM treated by radiosurgery without prior embolization (Andrade- Souza et al., 2007).
Cons
- Sometimes inadequate by itself to permanently obliterate AVMs,
- Induces acute hemodynamic changes,
- May require multiple procedures,
- Embolization prior to SRS reduces the obliteration rate from 70% (without embolization) to 47% (with embolization)
Agents
- Liquid agents: Onyx
- Ethylene-vinyl alcohol (EVOH) copolymer (ethylene and vinyl alcohol) dissolved in dimethyl sulfoxide (DMSO) with micronised tantalum (for radio-opacity).
- Not an adhesive → greater control with release → the best.
- Onyx-18 corresponds to the viscosity
- Onyx-18, Onyx-34 (both for AVMs)
- Onyx-500 (for aneurysms).
- When in-contact with aqueous solution (blood, water) → Solidifies through precipitation
- Pathologic changes (similar to the acrylates) include: endothelial necrosis, acute inflammatory reaction, foreign body giant cells.
- Onyx is bright on T1WI MRI
- Particulates: polyvinyl alcohol (PVA) particles
- Nidus obliteration is slower than with liquid agents → before complete obliteration → nidus is exposed to inc. Pressure → theoratical inc. The risk of haemorrhage
- Acrylates: Adhesive
- Can accidentally glue catheter to artery
- Eg: NBCA (N-butyl cyanoacrylate)
Before using definitive treatment (surg or SRS)
- Surgery: wait 3 -30 days (??)
- SRS
- Wait 30 days → immediate post embo angio looks amazing and you can leave out parts of AVM during SRS planning
- Do not use radio-opaque material in embolization because will cause CT not useable for SRS planning
Delayed post embolization deterioration
- Haemorrhage
- Steal
- Retrograde venous thrombus
Outcome
- EVOH
- Disabling morbidity, mortality, and urgent surgery occurred in 6.6% of cases and complete ablation of arteriovenous shunting in 27% (Morgan et al., 2013)
- Morbidity was reported in 5.1%, mortality in 4.3% and AVM obliteration in 23.5% (Pierot et al., 2013).
- Risk of embolisation
- The cumulative freedom from haemorrhage following radiosurgery for SPC A bAVM is extrapolated from combining the natural history of haemorrhage after diagnosis derived from a meta-analysis, with the proportion that is not obliterated as reported from Kano and colleagues.
- See Gross 2013
Radiation treatment
Conventional radiation
- Effective in ≈ 20% or less of cases.→ not an effective therapy
Stereotactic radiosurgery (SRS) : accepted for some small (≤ 2.5-3 cm nidus), deep AVMs
- 3 years, 3cm
- Indicated
- Deep inaccessible lesion
- Lesion close to eloquent cortex
- Mechanism
- Radiation damages DNA in rapidly dividing endothelial cells → endothelial cells attempt to regenerate → they are depleted over time → eventually Intimal disintegration occurs → exposes smooth muscle cells and triggers a proliferative response in the medial layer → progressive growth of the media thickens the arterial wall and constricts the lumen → eventual vessel occlusion.
- Technique
- Leksell gamma knife
- Treatment planning based on
- MRI
- Angiography
- Both
- Dose
- 18 Gy or more
- The radiation dose prescription was based on the risk of developing radiation-related complications, as predicted by the integrated logistical equation
- AVM margin dosesAVM volume cm325 Gy220 Gy2-418 Gy4-816 Gy8-12<16 Gy rare12
- The smaller the volume of AVM irradiated the steeper the fall off in dose of radiation delivered to the surrounding brain
- Pros
- Done as an outpatient,
- Non-invasive,
- Gradual reduction of AVM flow,
- No recovery period
- Cons
- Takes 1-3 years to work (during that time there is a risk of bleeding, controversial whether it is increased or decreased)
- Limited to lesions with nidus ≤ 3 cm
Outcome: Wegner 2010 determined at the patient’s last follow-up review
Excellent outcome | Complete nidus obliteration and development of no new neurological deficits. |
Good outcome | AVM obliteration was also achieved but was associated with the development of a minor deficit (e.g., quadrantanopsia, ataxia, or cranial nerve injury) that did not interfere with the patient’s normal level of activities |
Fair outcome | Obliteration was achieved but the patient developeda major deficit (e.g., hemiparesis, aphasia, or homonymous hemianopsia) that resulted in a decline in his or her level of functioning. |
Unchanged | If follow-up imaging confirmed persistent arteriovenous shunting but had no new neurological deficits. |
Poor outcome | Any patient who developed a new neurological deficit but had incomplete nidus obliteration |
- Nidus obliteration
- Total nidus obliteration rate (on MRI/DSA) was documented in 198 patients (68%)
- Median time to obliteration was 35 months
- Complication
- 10 (53%) died
- 9 survived
- 1 had a new permanent deficit from the bleeding event.
Developed permanent radiation related neurologic deficits | 13% |
Haemorrhage during the latency interval after radiosurgery | 6.5% |
Maruyama 2005 et al
- 11.5%/year bleed rate before SRS
- 4.2%/year bleed rate after SRS but prior to complete obliteration
- 0.6%/year bleed rate after complete obliteration post SRS
- Calculation
Combination techniques
- e.g. Embolization to shrink nidus then stereotactic radiosurgery