Define
- AVF draining to median prosencephalon vein of Markowski (a precursor of vein of Galen)
- congenital malformation that develops during weeks 6-11 of fetal development as persistent embryonic prosencephalic vein of markowski.
- Prosencephalic veins drain into the vein of Galen.
Number
- Rare anomalies of intracranial circulation
- Constitute 1% of all intracranial vascular malformations
- Represent 30% of vascular malformations presenting in the paediatric age group
- Incidence: 1/3mil population per year
Embryology
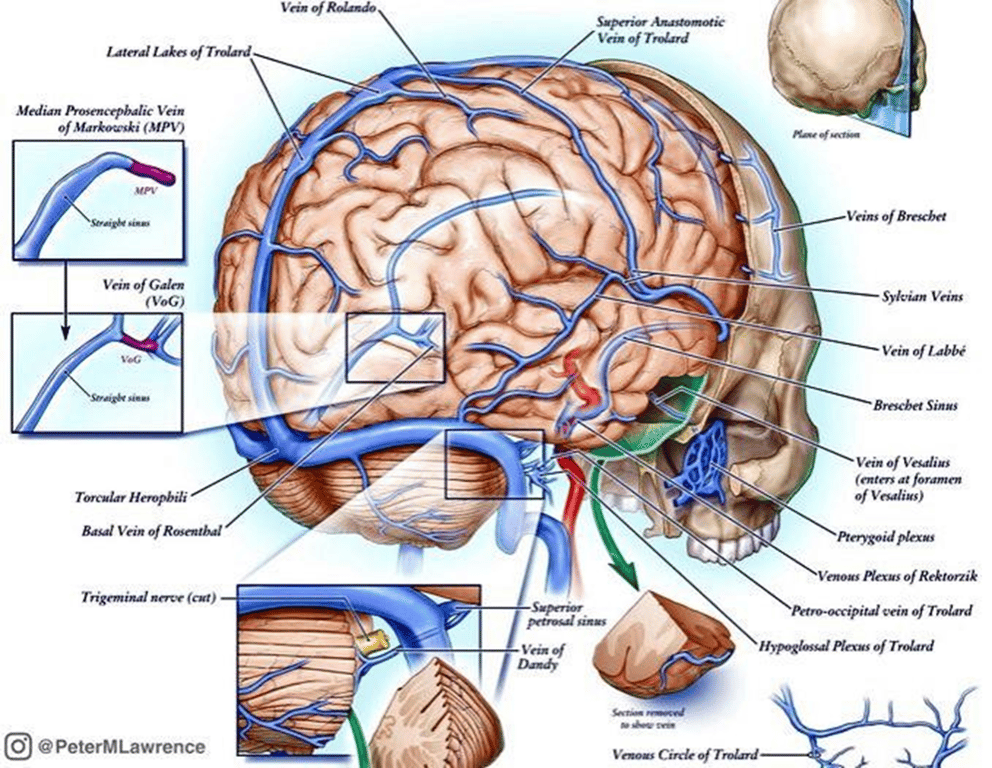
Normal development of dorsal cerebral vasculature
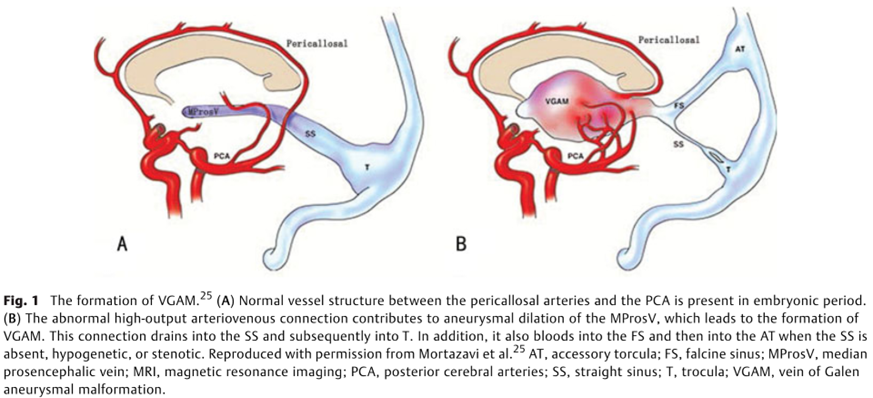
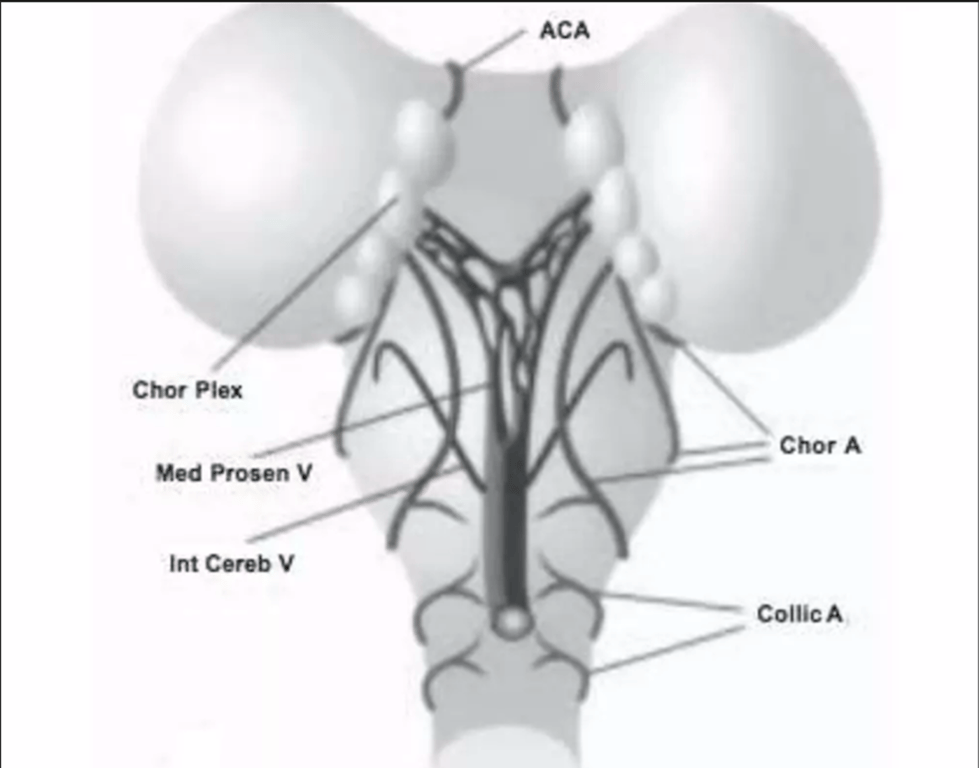
- The Choroid Plexus is
- supplied by the
- Anterior cerebral (ACA)
- Choroidal arteries (Chor A)
- Drains into the
- median prosencephalic vein (Med Prosen V)
- Development of the internal cerebral veins (Int Cereb V) results in the regression of the median prosencephalic vein
- Disease
- This abnormal development occurs between 6-11 weeks of intrauterine life
- Location of the AV fistula is within the cistern of velum interpositum and quadrigeminal cistern
- Arteriovenous fistulous communications prevent regression of the median prosencephalic vein
- Ectatic venous structure characteristically seen in the lesion represented the median prosencephalic vein and not the vein of Galen itself
- Arise as a result of direct arteriovenous communications
- between
- Arterial network
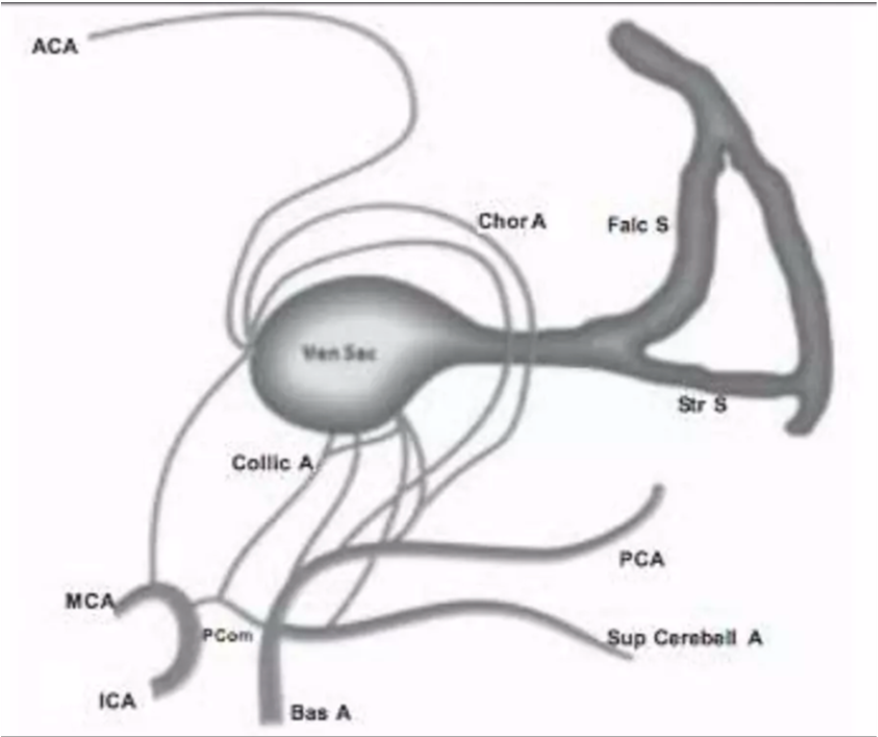
- Principal feeders of malformation are those that normally supply the tela choroidea and the quadrigeminal plate including:
- The anterior or prosencephalic group
- Anterior cerebral (ACA)
- Anterior choroidal (Chor A)
- Middle cerebral (MCA)
- Posterolateral choroidal arteries
- The posterior or mesencephalic group
- Posteromedial choroidal
- Posterior thalamoperforating
- Quadrigeminal
- Superior cerebellar arteries (Collic A)
- median prosencephalic vein
- lacks a fibrous wall therefore is unsupported --> can balloon out to a large size
- Ectatic venous structure characteristically seen in the lesion represented the median prosencephalic vein and not the vein of Galen itself
- lies free in the subarachnoid space within the cistern of velum interpositum
- Venous drainage
- into the
- Falcine sinus (Falc S)
- high flow across the arteriovenous fistula may result in retention of foetal patterns of venous drainage
- Persistence of falcine sinus, which is supposed to be a transient embryonic structure that connects the straight sinus to the superior sagittal sinus
- hypoplasia of the straight sinus (Str S)
- Retention of foetal patterns of venous drainage (falcine sinus) could prevent development of other sinuses such as the straight sinus.
- Retention of the embryonic pattern of vasculature can explain the presence of the several vascular anomalies associated with the VOG malformation.
- Aneurysmally dilated midline deep venous structure, fed by abnormal arteriovenous communications
Classification
Lasjaunias classification: by number and origin of feeding vessels
Feature | Mural | Choroidal |
Number of Fistula | Few or single | Multiple fistula |
Entry Point | Enter prosencephalon vein through the wall of the median prosencephalic vein | Enters prosencephalon vein at the anterior aspect of the median prosencephalic vein |
Feeders | Quadrigeminal arteries Post. Choroidal arteries | Ant and post. Choroidal arteries Pericallosal arcade (from Anterior cerebral artery) Thalamoperforating artery |
Flow | High flow but lower than choroidal | Highest flow |
Age of presentation | Present later (infant) | Neonates |
Presentation | Large fistula —> High output cardiac failure Smaller fistula —> hydrodynamic syndrome | |
Dilatation | More rounded dilatation than Choroidal | - |
Associated Anomalies | Absence or stenosis of dural sinuses, Stenosis at the level of the jugular foramen | Often has artery to artery anastomoses before fistulating |
Tx | Less embolization needed to achieve occlusion | More embolization needed to achieve occlusion |
Overall prognosis | Better | Worse |
Image
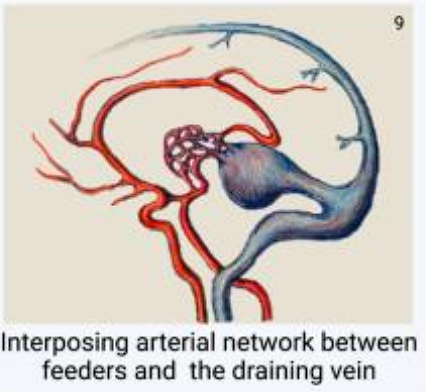
Based on location of the fistula (Yasargil’s)
- Pure internal fistulae: single/multiple
- Fistula between thalamoperforators and the VOG
- Mixed form: most common
- Plexiform AVMs
Pathology
flowchart LR linkStyle default stroke:White,stroke-width:4px Neonate --> Infant --> Child1 --> Child2 subgraph Neonate [Neonate] direction TB style Neonate fill:#a96648 A[Congestive Heart Failure] --> A1[Multiorgan Failure, encephalomalacia] end subgraph Infant [Infant] direction TB style Infant fill:#854e33 B[Macrocrania, Hydrocephalus] B --> B1[Dural Sinus Thrombosis] B1 --> B2[Dural venous congestion and supratentorial pial reflux, Bone hyperthrophy] --> B7[Facial venous collarteral, Epistaxis] B2 --> B8[Convulsion, Neurological deficits, ICH] B1 --> B3[Infratentorial pial reflux and congestion] B3 --> B4[Tonsilar Herniation]--> B5[Cerebellar and Brainstem Compression] B4 --> B6[Syringohydromyelia] end subgraph Child1 [Child <5 years] direction TB style Child1 fill:#6d3918 C[Neurocognitive Delay] end subgraph Child2 [Child >5 years] direction TB style Child2 fill:#58300a D[ependymal atrophy] --> D1[Pseudo-ventriculomegally, calcification] --> D2[Epilepsy, neurological deficits] end
- Cardiac manifestation: Neonates with VOG malformations the cardiac failure is multifactorial in origin and usually refractory to medical management
- High flow of blood through the fistula can lead to cardiac output
- Cardiac high output failure
- 80% of the left ventricular output may be supplied to the brain in severe cases.
- High flow across the pulmonary vasculature --> pulmonary hypertension
- High venous return to the right atrium promotes right-to-left shunting through
- Patent foramen ovale
- Ductus arteriosus
- Remains patent due to the rise of pulmonary arterial pressure above the systemic pressure.
- These right-to-left shunts are responsible for the cyanosis that may occur in these patients
- Cardiac ischaemia due to reduction in endocardial blood flow because:
- Arteriovenous shunts --> reduce the diastolic pressure within the aorta --> reduced coronary artery flow.
- Increased cardiac output results in high ventricular intracavity pressure
- Neurological
- Cerebral venous hypertension
- is the factor that is responsible for most neurological manifestations of VOG malformations.
- Due to
- high flow fistula
- Venous anomalies in the form of
- poorly developed venous drainage
- secondary venous stenosis and occlusion
- The high venous pressure transmitted to the medullary veins prevents resorption of fluid and thus results in
- Hydrocephalus
- Due to
- Impaired resorption of CSF
- In infants, the arachnoid granulations have not fully matured, so most of the ventricular CSF is reabsorbed across the ventricular ependyma, into the brain parenchyma, for subsequent drainage by the medullary veins
- Can also less commonly be obstructive from aqueduct compression
- Cerebral oedema
- Hypoxia
- from venous hypertension results in progressive cerebral parenchymal damage resulting in cognitive impairment, which can range from delayed milestones to mental retardation
- Others
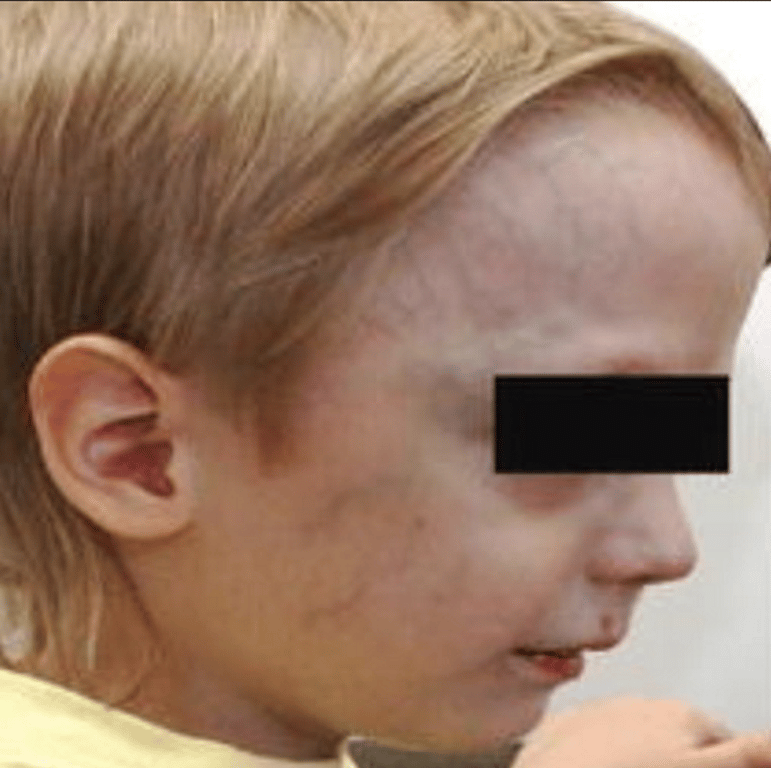
- Prominent facial veins (commonly seen in these infants) + epistaxis
- fistula may be drained by rerouting its flow into the cavernous sinus and further into the facial veins or basilar or pterygoid plexus.
Natural hx
- Untreated VOG malformations have a poor prognosis
- Neonates
- 100% mortality
- 1-12 months old:
- 60% mortality
- 7% major morbidity
- 21% normal
Clinical features
- Gold et al (1964): clinical classification system correlating age at presentation with the clinical presentation and pathophysiology and described three groups
- Neonates
- Multiple fistulas
- 25% of cardiac output passing through fistula causing high output cardiac failure
- Depending on various factors, the cardiac manifestations can range from asymptomatic cardiomegaly to severe cardiac failure that is refractory to medical management
- Cyanosis can be seen in these patients and mistaken for congenital cyanotic heart disease
- Cranial bruit and marked carotid pulses
- Bicetre neonatal evaluation score Lasjaunias 1997
- Children and infants
- Single fistula with smaller shunt
- Cardiac manifestations are absent or very mild
- Macrocephaly or with hydrocephalus
- Longstanding cerebral venous hypertension – delayed milestones
- High proportion – failure to thrive
- Due to Cardiac decompensation, hypothalamic and hypophyseal dysfunction secondary to venous congestion
- Older children
- Low flow fistulae
- Usually present with headache and seizures
- Small number also present with developmental delay, focal neurological deficits, proptosis and epistaxis
- SAH and ICH can also occur
Points | Cardiac Function | Cerebral Function | Respiratory Function | Hepatic Function | Renal Function |
5 | normal | normal | normal | ㅤ | ㅤ |
4 | overload, no medical treatment | subclinical, isolated EEG abnormalities | tachypnea, finishes bottle | ㅤ | ㅤ |
3 | failure; stable with medical treatment | nonconvulsive intermittent neurologic signs | tachypnea, does not finish bottle | no hepatomegaly, normal hepatic function | normal |
2 | failure; not stable with medical treatment | isolated convulsion | assisted ventilation, normal saturation Fi02 < 25% | hepatomegaly, normal hepatic function | transient anuria |
1 | ventilation necessary | seizures | assisted ventilation, normal saturation Fi02 > 25% | moderate or transient hepatic insufficiency | unstable diuresis with treatment |
0 | resistant to medical therapy | permanent neurological signs | assisted ventilation, desaturations | abnormal coagulation, elevated enzymes | anuria |
Maximal score = 5 (cardiac) + 5 (cerebral) + 5 (respiratory) + 3 (hepatic) + 3 (renal) = 21
Diagnosis
- US
- Antenatal US
- venous sac appears as a mass located posterior to third ventricle.
- Pulsatile flow within helps to differentiate.
- Visualize hydrocephalus, cardiac dysfunction
- Postnatal US
- Assess haemodynamic changes.
- Useful serial follow up in pts treated with endovascular therapy.
- CT
- Well defined multilobulated intensely enhancing lesion.
- Dilated vents.
- Periventricular lucency, diffuse atrophy.
- Diffuse ischaemic change
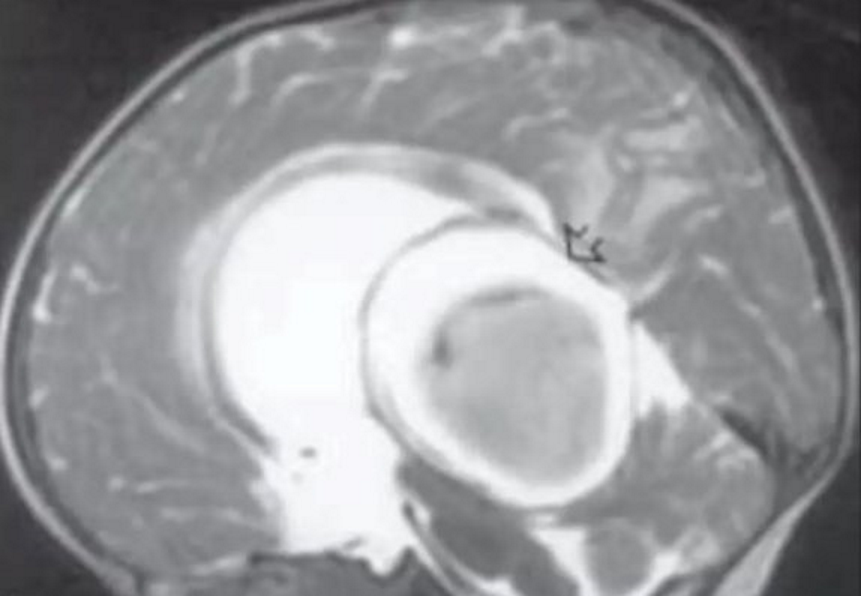
- MRI
- Useful to demonstrate location of fistula, presence of nidus, arterial components, venous sac and status of venous drainage
- Assess for thrombus within VOG malformation
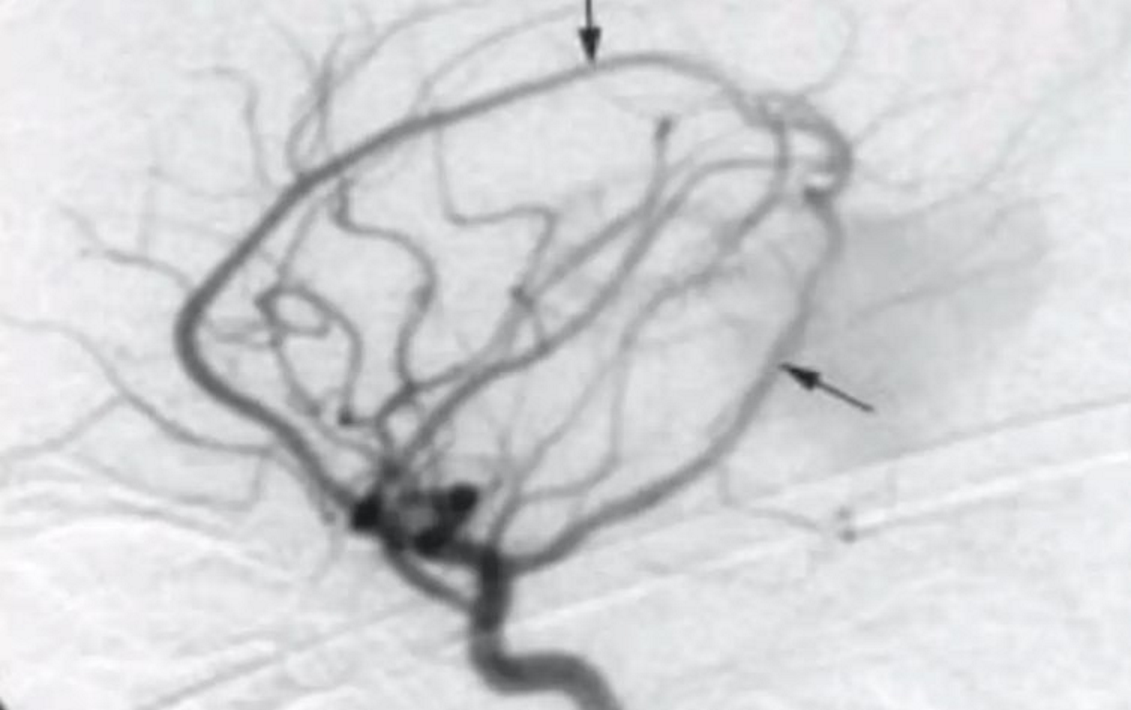
- Angiography
- Gold standard
- Better at demonstrating small feeders as well as dynamic aspects
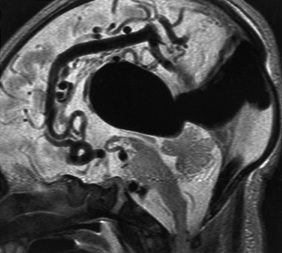
- Venous drainage of normal brain
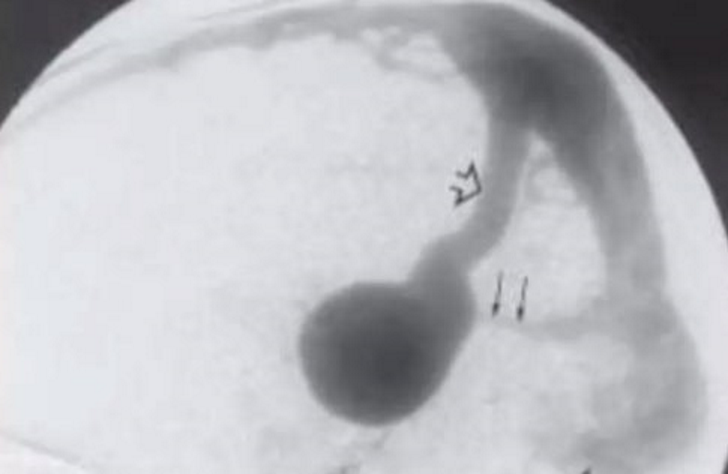
~Large arrow: persistent falcine vein
~Small arrow: hypoplastic straight sinus
~Small arrow: hypoplastic straight sinus
Management
- Hydrocephalus - VPS
- Indication
- If obstructive HCP - Requiring shunt. Risks with precipitating haemorrhage
- VOG malformations
- Limited efficacy of operative treatments for those in poor medical condition
- Untreated
- Very poor prognosis
- High proportion who present in neonatal period rapidly deteriorate and succumb to congestive cardiac failure
- Rapid/aggressive tx of cardiac failure is essential.
- Aggressive medical mx can usually postpone an intervention until child aged 5-6months – intervention easier and safer
- Emergency embolization of the malformation may be necessary to reduce the shunt in neonates with CCF that is refractory to medical therapy
- Surgery Vs Endovascular
- Surgical
- Issues with surgery
- Despite technological advances – complete elimination of lesion rarely achieved
- Major surgery
- Deep-seated
- High-flow shunt in infant with multiorgan failure compounded by poor myelination of brain parenchyma
- Parenchyma tears easily on retraction
- Shunting can worsen cerebral venous hypertension
- Aim to avoid before elimination of AV shunt
- Endovascular
- Aim
- to reduce the volume load initially
- to arrest the cardiac failure
- attempt to finally obliterate the shunt completely
- Indication
- Refractory cardiac failure
- Acute or symptomatic hydrocephalus
- Rapid neurological deterioration
- When parenchymal calcifications appear on follow-up scanning of brain.
- Not indicated in because
- poor clinical outcome in spite of successful closure of the shunt by embolization.
- Encephalomalacia
- Severe brain damage
- Severe parenchymal loss
- Technique
- If able to access femoral vein or artery
- Transarterial embolization
- Used when the feeding arterial branches from the choroidal and perforator arteries are big enough to permit microcatheters passage.
- Transvenous embolization
- Used when
- the perforating arteries are too small to permit microcatheters passage
- the shunt is very large with extremely high flow
- Venous approach is preferred to avoid migration of the embolic material when delivered by the transarterial route.
- Occasionally it is necessary to use a combination of both techniques.
- If unable to access femoral vein or artery
- Neonates: Occipital bone over the torcular is penetrated with a large bore needle for catheterization of the varix
- Children: Occipital burr-hole is used
- Staging of the embolization
- Required in most infants and children
- Ranging from a few weeks to a few months based on the angioarchitecture and clinical status.
- The follow-up endovascular approach is based on the residual shunt and the architecture of the malformation.
- If the intervals between embolization is too long can lead to Occlusive venopathy
- a well-known delayed event causing progressive neurological deterioration.
- The acquired venopathy may be fatal.
- Mech:
- too long intervals between the embolization procedures --> high venous pressures in the dural sinuses and cortical veins --> back pressure in the medullary veins and cortical veins --> progressive parenchymal calcifications +refractory seizures.
Complications
- Potentially fatal
- Normal perfusion pressure breakthrough
- Intracerebral haemorrhage due to venous hypertension
- Can be reduced/avoided by staging the embolization procedures
- Perforation of venous sac
- Ischaemic deficits
- Pulmonary embolisation with embolic agents is common considering the high flow across the intracranial shunt
Outcome