Definition
- Well circumscribed benign vascular hamartoma.
- Consisting of irregular thick and thin walled sinusoidal vascular channels located within the brain
- But lacking intervening neural parenchyma, large feeding arteries, or large draining veins
Aka
- Cavernous malformations
- Cavernous haemangiomas
- Cavernous angiomas
Numbers
- Most frequently present in the 40-50s
- Small preponderance in women (58%)
- incidence of CCMs varies between 0.17 and 0.56 per 100 000 population per year
- 10% of CNS vascular malformation
- 10% of paediatric spontaneous cerebral haemorrhage
- 0.6% of incidental MRI findings
- 10–20% of CCMs are associated with a developmental venous anomaly
- an extreme variation of the normal venous anatomy
- Prevalence between 0.16 percent and 0.9 percent,
- Familial form has a prevalence of 0.01 percent to 0.03 percent.
Localisation
Supratentorial | 60% |
Brainstem | 30% mainly pons |
Basal ganglia | 10% |
Spinal | Intramedullary, IDEM, extradural, osseous, extradural spinal canal, neuronal |
Pathophysiology (Snellings et al 2021)
Features | Sporadic | Hereditary |
Fq | 50% | 20-50% |
Genes | MAP3K3 mutation | KRIT1>malcavernin/PDCD10 |
Multiplicity of lesion | more likely (80-90%) | Less likely 10-20% |
Haemorrhagic risk | Higher | Lower |
Association with DVAs | Lower | Higher |
Radiation exposure | Yes | No |
Congenital
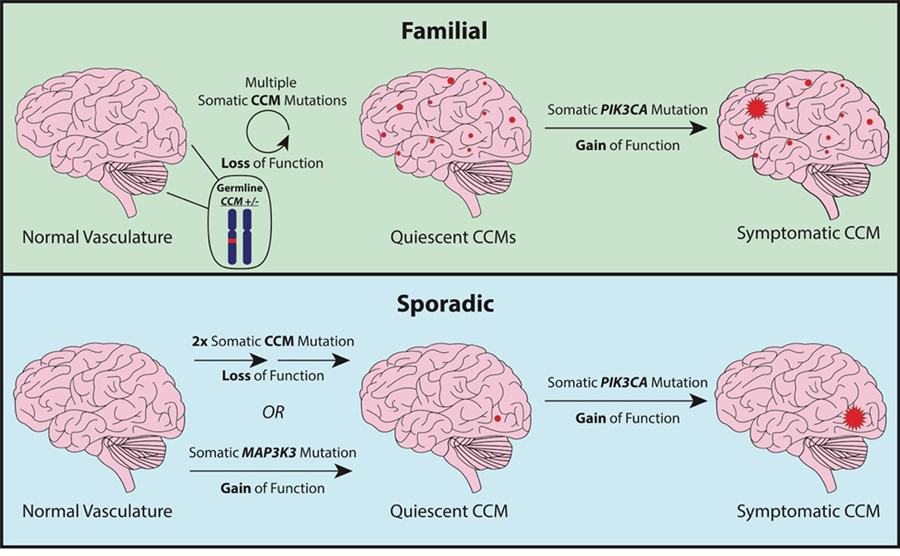
- A subset of these CCMs acquire a somatic gain of function mutation in PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) which fuels lesion growth.
Type | Gene | Chr | Possible function(s) | Notes |
CCM1 | KRIT1 (k-rev interaction trapped protein 1) | 7q11.2-21 | Angiogenesis, Inhibit endothelial cells, Apoptosis, Migration | Have other cutaneous vascular lesions Hispanic-American patients of Mexican descent, the proportion of familial cases may be as high as 50% due to a shared mutation in the KRIT1 |
CCM2 | MGC4607 Malcavernin | 7p13 | Stabilize endothelial cell junctions Maintain endothelial integrity | More likely to be asymptomatic have lower number of cavernomas |
CCM3 | PDCD10 (programmed cell death 10) | 3q26.1 | Stimulate cell proliferation regulate angiogenesis Vasculogenesis Regulate apoptotic pathway | More aggressive presentation (greater number of lesions, Scoliosis,, Meningioma, and more frequent haemorrhage and presentation in childhood) |
Unspecified gene | ㅤ | 3q26.3-27.2 | ㅤ | ㅤ |
- Autosomal Dominant
- but with variable of penetrance
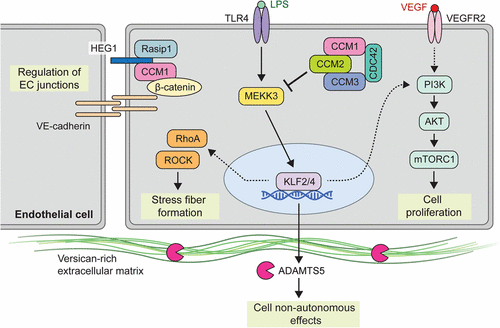
- Proteins encoded by these CCM genes
- are implicated in junction formation between endothelial cells in vessels.
- Loss-of-function mutations may result in abnormal blood vessels with gaps between these endothelial subunits
- Presentation
- Retinal cavernous malformation
- Skin venous malformation
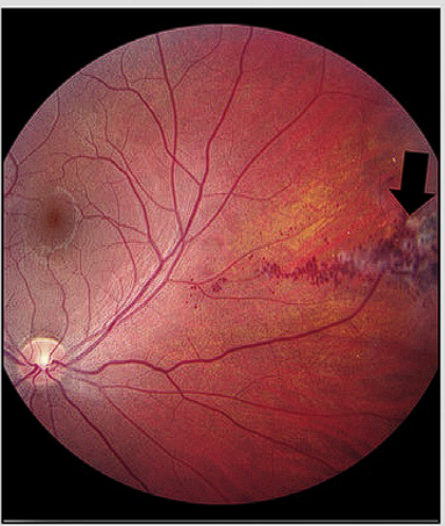
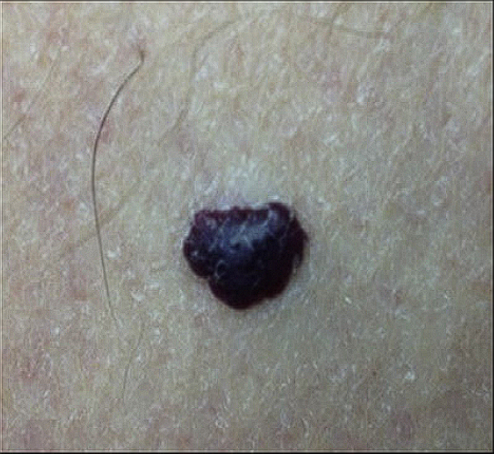
- Screening
- Screen if
- adults with CCM(s) + FHx of CCM,
- adults with multiple CCM + no FHx.
- Reasons
- Adult with a single CCM but no family history: 0% to 1%
- Adult with multiple CCMs but no family history: 57%
- Adult with at least one CCM and a family history: 78% to 94%
- See Wilson screening criteria
De Novo formation
- Radiation exposure
- With latent period of 9 yrs
- Immature brain more susceptible
- With local gene mutation
- Vessel wall changes due to radiation
Radiation induced Sporadic CCM | Non-radiation induced sporadic CCMs |
pathologically similar | pathologically similar |
multiple lesions | solitary lesion. |
Younger age at time of radiation treatment | ㅤ |
higher doses of ionizing radiation | ㅤ |
Pathology
Macroscopic
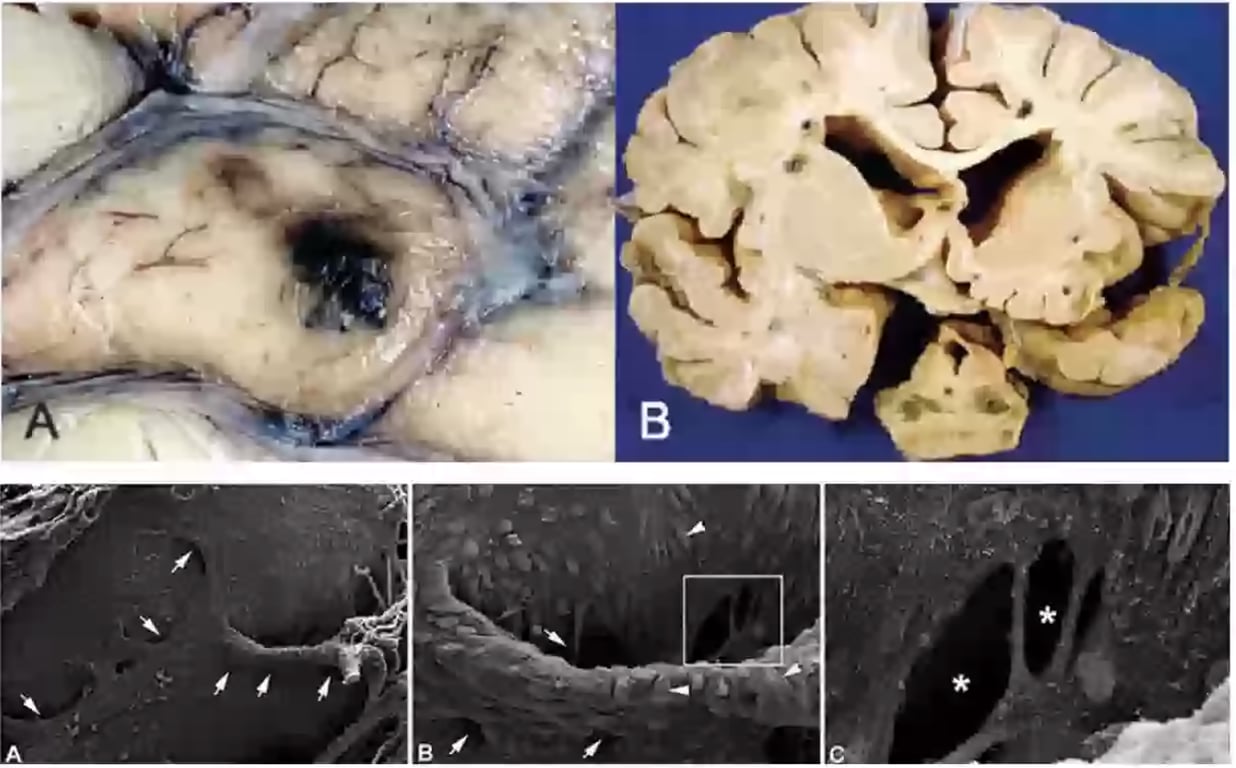
- Size: 1-5cm
- Circumscribed and lobulated with a reddish-purple raspberry appearance
- Surrounding brain tissue often contains haemosiderin.
Microscopic
- Cavernous malformations show sinusoidal congested vessels arranged back-to-back with little or no interposed brain tissue
- Multiple in 50% cases
- Can haemorrhage, calcify, thrombose
- Pathology "Mulberry lesion": cerebral haemorrhoids
- Radiology: popcorn lesions
- Light MS: Stains for vWB factor
- Blood vessels devoid of muscular and elastic tissue that are lined with endothelial cells that do not have intervening tight junctions.
- Electron MS:
- Abnormal gapping of tight junction between endothelial cells → leakage of blood
- Rare but can form in spinal cord. Associated with spinal radiotherapy
- Associated with venous angiomas → do not surgical remove them THEY BLEED
- Caverns are filled with blood in various stages of thrombus formation → organisation → dissolution
- Haemosiderin deposition and gliosis are found in abutting parenchyma.
- Characteristic haemosiderin depositions are present in the basal lamina and intervening neural tissue is absent, which differentiates them from capillary telangiectasia
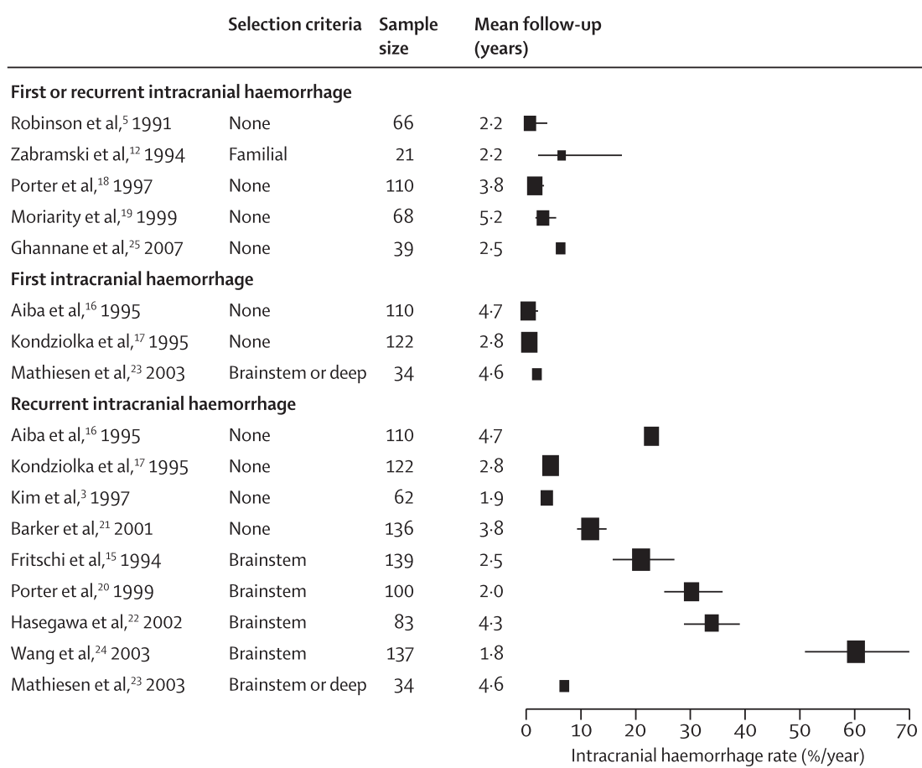
Natural history of Cavernomas
- General: issues with natural history studies
- Describes selected patients who did not receive treatment for some reason
- Describes a short untreated follow-up of those who did receive treatment.
- Large majority of those studies are hospital-based and have not looked at the population in general.
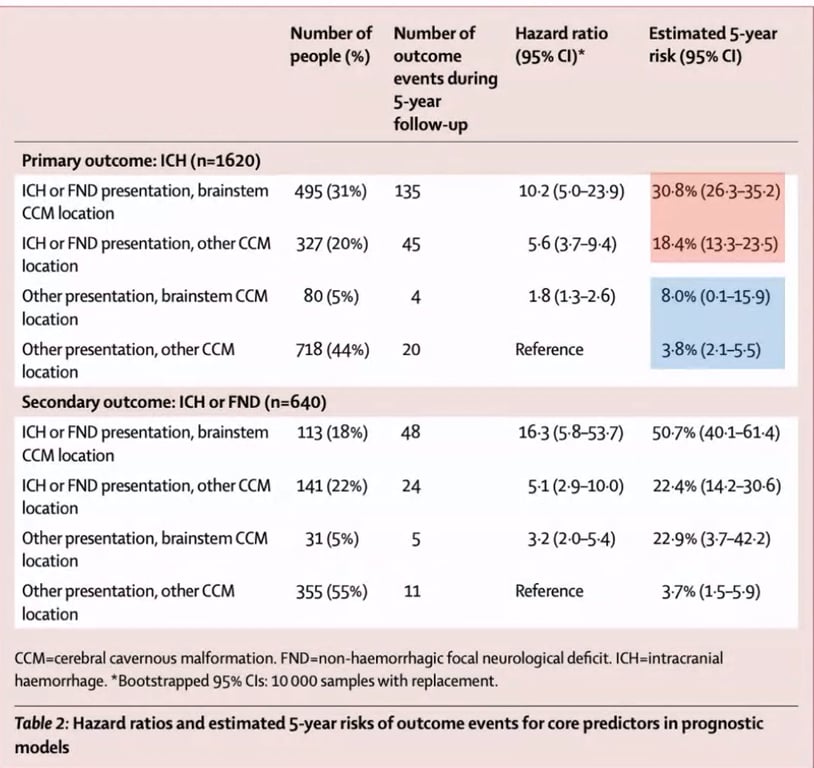
- Horne et al 2016: meta-analysis of hospital + population based study
- Higher risk of bleed in brainstem than other locations
- Higher risk of presenting with ICH or new focal neurological deficits than other presentation methods
- 5 yrs risk of ICH if left un tx
- 50% of the CCM patients become two years seizure-free within 5 years following the diagnosis of epilepsy
ㅤ | Without initial presentation of ICH/FND | With initial presentation of ICH/FND |
Non brainstem CCM | 3.8% | 18.4% |
Brainstem CCM | 8.0% | 30.8% |
Modified for memorization
Annual risk | Without initial presentation of ICH/FND | With initial presentation of ICH/FND |
Non brainstem CCM | 1% | 4% |
Brainstem CCM | 2% | 8% |
- Al-Shahi Salman et al. 2012: Population study & Flemming et al. 2012
- Annual risk of first-ever CCM-related haemorrhage
- Low (0.4–0.6% per year)
- Annual risk of a subsequent haemorrhage
- much higher (3.8–23% per year)
- This risk decreases over time
- Variable annual haemorrhage rate of
- 6.2% in patients who presented with a bleed
- 2.2% in patients presenting with non-haemorrhage related symptoms and
- 0.33% in patients with incidental CCMs
- Presentation with haemorrhage, male sex and multiplicity of lesions can increase the haemorrhage risk
- Taslimi 2016: Meta-analysis of 25 studies
- Hemorrhage definition
- Acute or subacute onset of symptoms accompanied by radiologic, pathologic, surgical, or CSF evidence of recent extralesional or intralesional haemorrhage
- Annual haemorrhage rates per person:
- 0.3% non-brainstem
- 2.8% brainstem
- Annual RE-haemorrhage rates per person:
- 6.3% non-brainstem
- 32.3% brainstem
- median 10.5 months to rehaemorrhage
Classification
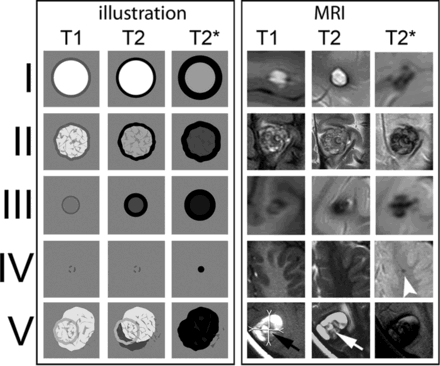
Zabramski classification of cavernoma
Lesion Type | MRI Signal Characteristics | Pathologic Characteristics | Adult overall 3 year MRI evidence of haemorrhage (Jeon et al 2013) |
Type I Subacute | T1: hyperintense core T2: hyper- or hypointense core with surrounding hypointense rim | Subacute hemorrhage, surrounded by a rim of hemosiderin-stained macrophages and gliotic brain | 9.47% |
Type II | T1: reticulated mixed-signal core T2: reticulated mixed-signal core with surrounding hypointense rim | Loculated areas of hemorrhage and thrombosis of varying ages, surrounded by gliotic, hemosiderin-stained brain; in large lesions, areas of calcification may be seen | 4.74% |
Type III Chronic | T1: iso- or hypointense core T2: hypointense with a hypointense rim that magnifies the size of the lesion GE: hypointense with greater magnification than T2 | Chronic resolved hemorrhage, with hemosiderin staining within and around the lesion | 1.43% |
Type IV | T1: poorly seen or not visualized at all T2: poorly seen or not visualized at all GE: punctate hypointense lesions | Multiple lesions in the category were pathologically documented as telangiectasias | ㅤ |
Differential diagnosis
- Hypointensity on hemosiderin-sensitive sequences Haemorrhagic metastases
- Cavernous malformation (type IV) Granuloma
- Calcium Infectious/inflammatory nodules
- Thrombosed arteriovenous malformation
- Microbleed due to cerebral amyloid angiopathy
- Calcified primary brain tumor
- Microbleed due to hypertension
- Trauma
- History of radiation to the brain
- Foreign body: metal/glass
- Cardiac surgery
Presentation
Incidentally (25-40%)
- Esp: with increase with MRI
- Asymptomatic is it because they
- haemorrhage in a non eloquent area OR
- they don't haemorrhage at all
Seizure 60%
- Due to inflammation, perilesional hemosiderin and gliosis
- Most common for frontal and temporal lobe cavernomas
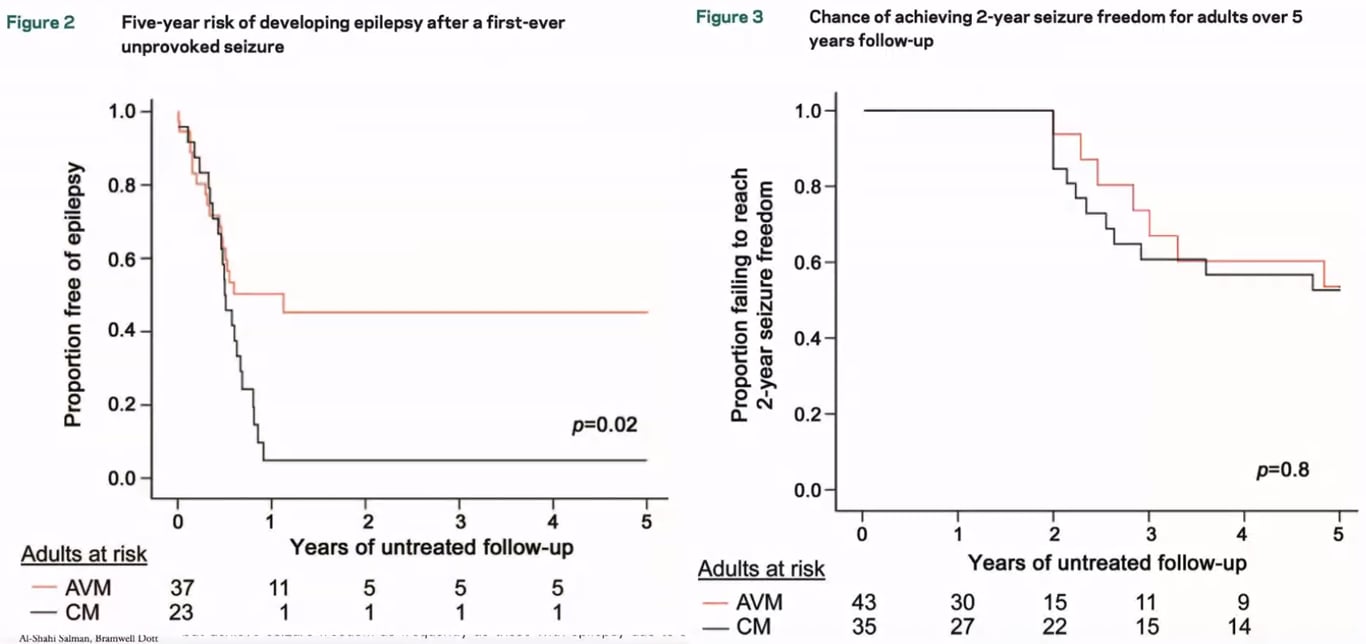
- Josephson 2011: 5-year risk of first time seizure in cavernoma patients
- 6% symptomatic
- 4% incidental (annualised: 0.9%)
- Higher risk of epilepsy in Cavernomas but can be well controlled with antiepileptics
- In patients with a single seizure, the risk of a recurrent seizure is as high as 94% over 5 years;
Progressive neurological deficit 50%
- Dependent on location of cavernoma
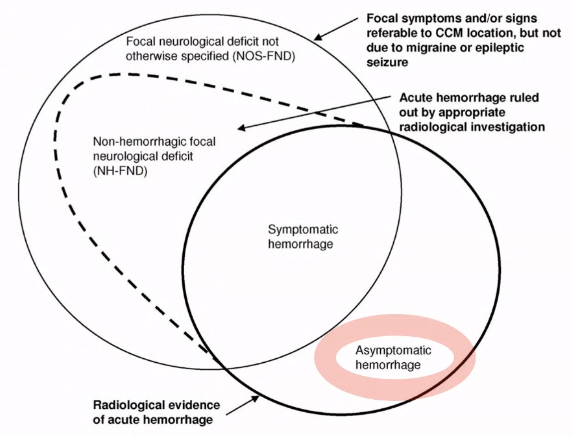
Haemorrhage 20% → only for symptomatic radiologically proven
- Definition: a clinical event with acute/subacute onset of symptoms with radiological, pathological, surgical or CSF evidence of either recent extralesional or intralesional haemorrhage
- Almost all cavernomas that have a hemosiderin around it has had a small leak
- Most haemorrhage outcome is good because it is small
- Risk of haemorrhage in cerebral CMs (Akers 2017)
- Risk of first-time haemorrhage among incidentally discovered CMs is very low (0.08%)
- 2 factors most consistently associated with increased bleeding risk
- Horne 2016
- hazard ratio = 5.6 (Horne 2016)
- 5 yr risk of bleed ICH: 18.4% (Horne 2016)
- Salman 2012 lancet
- 5 yr risk of bleed for 1st ICH: 2.4%
- 5 yr risk of bleed for recurrent ICH: 29.5%
- Salman 2012 summary of all paper
- Annual risk of 1st ICH: 0.3-0.6%
- Annual risk of recurrent intra-cranial haemorrhage: 3.8-22.9%
- Familial cases have higher risk
- hazard ratio = 4.4
- Salman 2012 summary of other paper
- 21-60.2% annual risk
- Inconsistent findings reported for risk differences with female gender, CM size, and CM multiplicity
- Annual risk of recurrent haemorrhage declines over time (Horne 2016)
- 6.2% (1st year) —> 2.0% (5th year)
- Higher annual ICH rates are reported in familial CMs (4.3–6%) than in sporadic cases but this could be artefactual
- Pregnancy & parturition are not thought to be risk factors for haemorrhage
- Risk of haemorrhage from a CM may not be increased by platelet inhibitors or anticoagulation but this is based on uncontrolled studies that likely avoided treatment of patients with recent haemorrhage—> don’t give them anticoagulation if can
- No relation of physical activity to haemorrhage from CMs has been identified
Previous haemorrhage: CMs initially presenting with haemorrhage:
Location of haemorrhage: brainstem CMs:
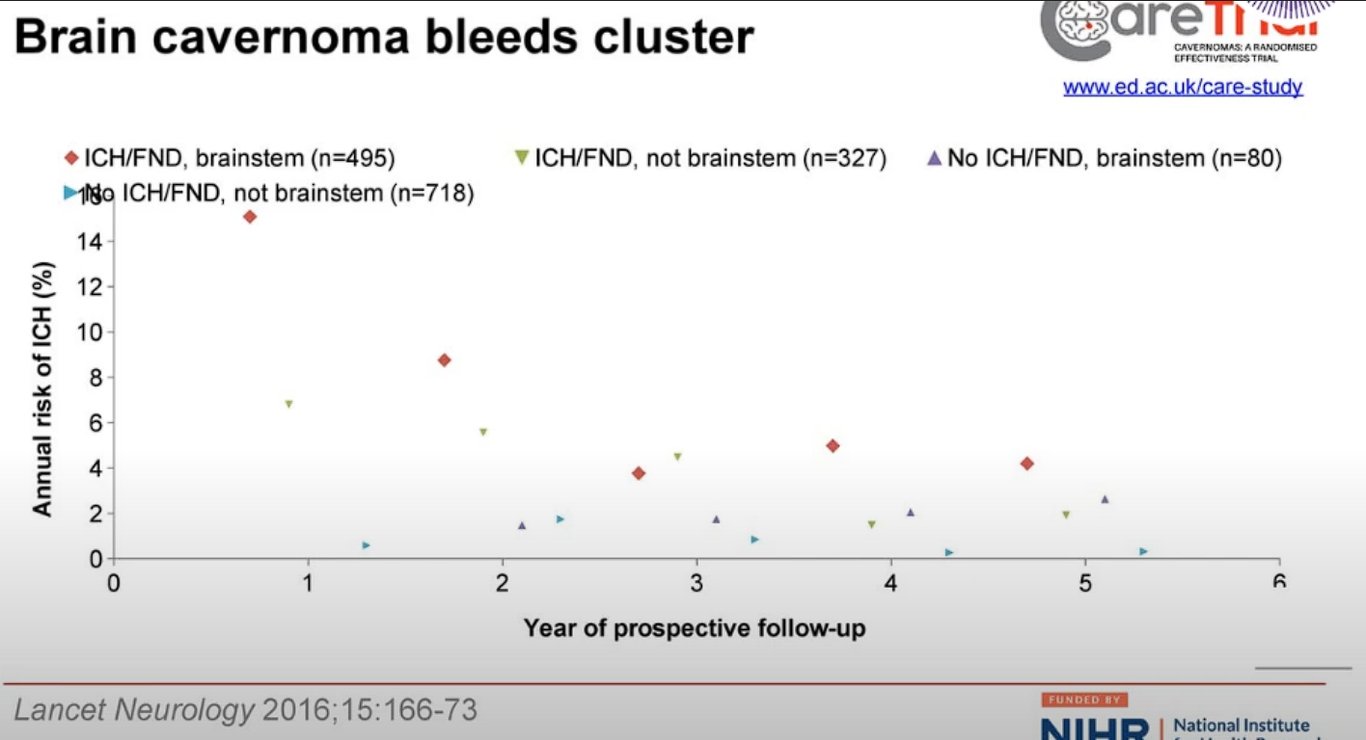
- Paediatric cavernomas (<25 yrs old)
- Do not bleed more often than adults
- Al-Holou 2011: n=92 pt with n=110 cavernomas
- Haemorrhage rate
- In general
- 1.6% per patient-year
- 0.9% per cavernoma-year
- Symptomatic group
- 8.0% per patient-year
- incidental group
- 0.2% per patient-year
- Previous bleed: higher risk of bleeding
- Younger age of bleeding in the familial population
Hydrocephalus
Radiology
- 19 to 31% are radiologically occult
- CT:
- not sensitive—> miss small lesions
- Poor mass effect
- 15% has calcification
- Higher epileptogenicity
- MRI:
- Gradient echo T2 most sensitive
- Popcorn: mixed signal core with low signal rim
- There is a difference in MRI features based on presence and absence of bleed and the age of the bleed
- Rim of hypointensity can extend and stain the adjacent brain parenchyma (blooming effect) --> making an fake larger lesion
- SWI
- Makes it very much clearly and with greater blooming effect
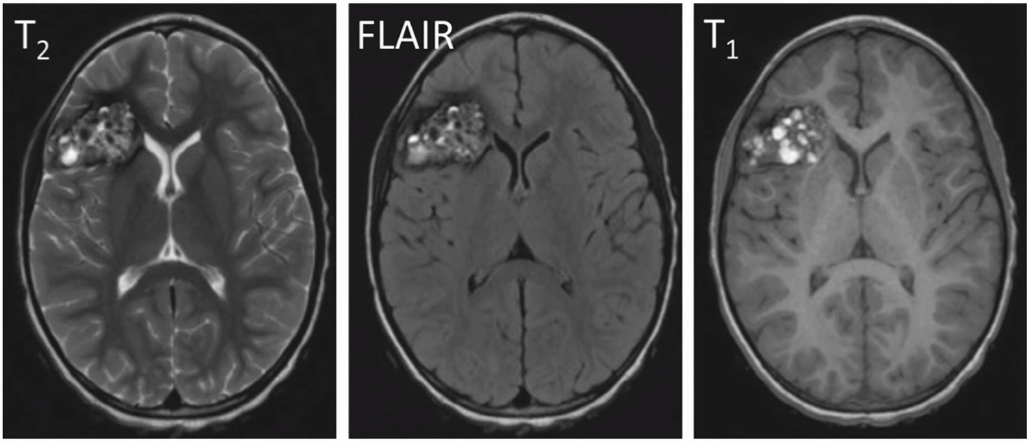
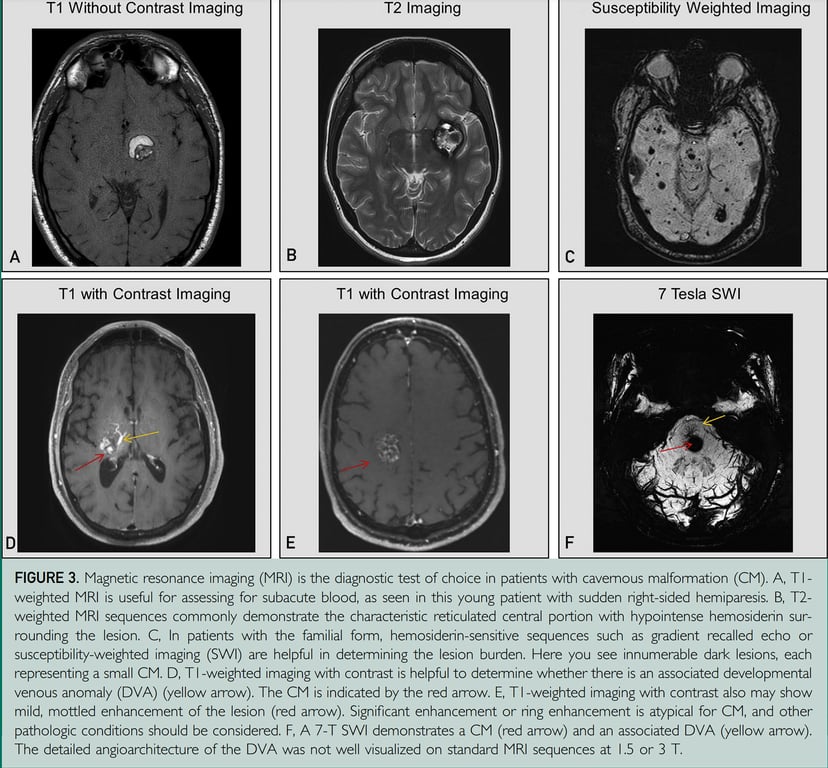
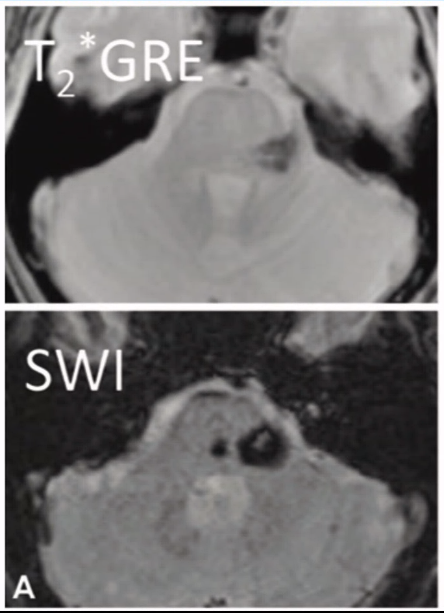
- Angiography:
- Does not demonstrate lesion
- Hence called angiographically occult lesions, or cryptic vascular malformations
- Unless has anomalous venous malformation
Management
Conservative management
- Indicated for
- Incidental cavernomas
- Symptomatic CCMs that are associated with high treatment risks, such as those in eloquent locations (e.g. brainstem)
- Repeat MRI 6 monthly - annually for 3 years
- Medication
- Propranolol use for familial cerebral cavernous malformation
- Aim
- lower risk of intracerebral haemorrhage
- Lower risk of new persistent or progressive focal neurological deficit
- Evidence
- Lanfranconi 2023 Pilot Treat_CCM trial
- Propranalol vs standard care N=83
- The incidence of symptomatic intracerebral haemorrhage or focal neurological deficit was
- propranolol = 1·7 cases per 100 person-years
- standard care = 3·9 cases per 100 person-years
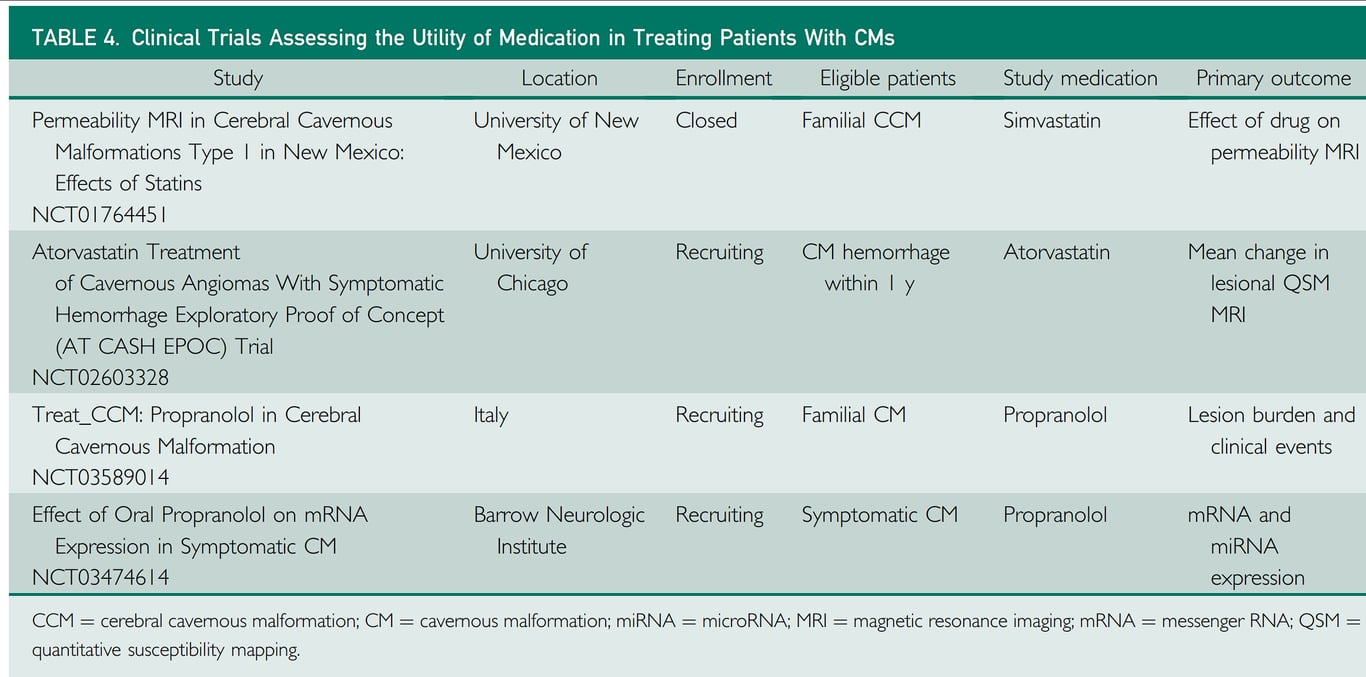
- Studies comparing Intervention vs conservative
Intervention
- No observational studies comparing microsurgical resection with radiosurgery
- Overall incidence of death, haemorrhage or new/worsened permanent deficit after resection or SRS, is approximately 6 per 100 person-years.
Microsurgery
- Indications
- Symptomatic cavernomas in non eloquent areas or brainstem
- multiples haemorrhages,
- CCMs with mass effect causing symptoms, and
- patients with intractable epilepsy with an identified epileptogenic focus
- If really need to go to brainstem look for the following factors
- Cavernoma should reach the pial or ependymal surface, in order to minimize operative neurological deficits
- Use relatively safe entry zones
- Use intraoperative electrophysiological
- Goal:
- complete excision of malformation
- If for seizures excision of surrounding haemosiderin brain)
- Technique
- Surgery planning
- Aim
- Find shortest trajectory to cavernoma
- Avoiding ‘blind-spots’ along the surgical corridor.
- Done by
- Utilising an extended line to the skull that incorporates one point in the centre of the CCM and a second point at where the CCM manifests at the pial surface
- Identification of Cavernoma
- Yellow discolouration
- Neuronavigation
- For non eloquent Cavernomas
- Circumferential dissection of the glial plane, emptying of the caverns to achieve internal debulking with subsequent facilitation of resection, by rolling it away from the glial plane.
- Ideally, an en-bloc resection should be attempted, since this minimizes the risk of incomplete resection.
- For eloquent CCMs
- requires an entirely different approach.
- An ‘inside-out’ dissection is preferable, which is done by entering and emptying the CCM to make it collapse on itself.
- This is followed by careful dissection of the plan between capsule and eloquent brain tissue to minimize surrounding brain injury
- If the CCM is associated with a DVA, the latter should be preserved during resection as it represents part of the normal venous system.
- Only small branches of the DVA can be cauterized but sacrifice of the main trunk(s) may lead to venous infarction with potentially disastrous consequences
- Outcome
- Poor prognostic factors after surgery
- Brainstem CCM
- Increasing age
- Male sex
- Kivelev et al. 2011
- Outcomes according to a grading scale
- Location
- 1 point: Supratentorial
- 2 points: Infratentorial basal ganglia, or spinal
- Pre-existing neurological deficit (1 point if present).
- A good functional outcome (Glasgow outcome score 5: normal activities, minor neurological deficits) was achieved in
- Grade 1: 87%
- Grade 2: 79%
- Grade 3: 46%
- Seizure freedom in CCM patients with medically refractory epilepsy can be allegedly achieved approximately 88% (seizure freedom for 80% of patients with chronic epilepsy due to CCMs and 91% CCM patients with sporadic seizures-von der Brelie et al., 2013).
- Depending on the definition of seizure and what defines seizure freedom
- Moultrie 2014
- Non randomized group study
- Surgery is worse than conservative management for both the risk of first haemorrhage or Focal neurological deficit and oxford handicap score
- Surgical risks were lower if
- patients presented with haemorrhage or
- in patients with CCMs outside the brainstem
- Mortality was comparable;
- Surgery group: one patient (4.0%) in the surgical group died after a seizure
- Conservative group: four patients (3.7%) experienced CCM-related deaths
- Surgery brings forward all the risk of CCM if completely excised when compared to conservative
- With further follow-up, the difference between conservative and surgery might diminish because of new neurologic events in the conservatively managed group, but this may not turn out to be the case because the risk of recurrent CCM haemorrhage seems to decline over time and CCM sometimes regrow after surgery and bleed again.
- Risk of postoperative complications (composite of death within 30 days, symptomatic haemorrhage, new or worsened focal neurological deficit) was estimated at
- 7.7% for studies looking at resection of predominantly supratentorial CCMs
- 50% for resection of brainstem CCMs
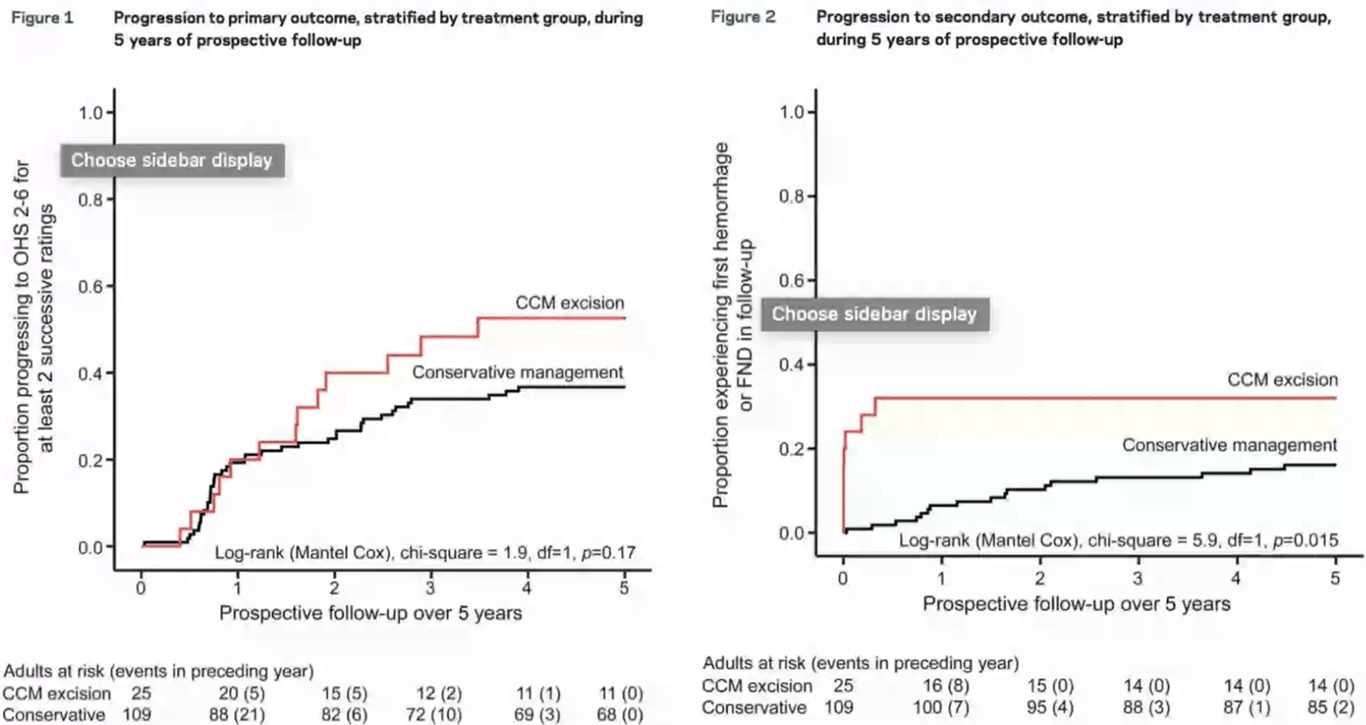
SRS
- Aim of SRS: prevention of haemorrhage by inducing thrombosis-obliteration in the cavernoma.
- Indicated
- Highly eloquently or surgically inaccessibly areas, such as basal ganglia and brainstem (where the cavernoma does not present at the surface).
- Small <3cm
- Seizure control
- CI
- Radiosurgery not advised in familial CCM
- Concern of proliferation of further CMs as RT in general can cause CCM
- Controversial as outcome seem similar to placebo
- Poorthuis 2019
- After SRS for CCM, the annual incidences of death, ICH, and FND are <5% and seem comparable to outcomes without SRS.
- As the risk of recurrent intracranial haemorrhage declines over time (Al-Shahi Salman et al., 2012; Flemming et al., 2012) it is questionable if the reduced haemorrhage risk after SRS that some studies have described can be attributed to this treatment
- As CCMs are angiographically occult lesions, the effectiveness of SRS cannot be assessed by imaging as in AVMs, but relies solely on the basis of occurrence of haemorrhage during follow-up period
- CARE Pilot RCT
- Arm
- (n33) medical management + surgery (Resection or SRS) VS
- (n34) medical management alone
- Primary clinical outcome
- Symptomatic intracranial haemorrhage or new persistent or progressive non-haemorrhagic focal neurological deficit due to cerebral cavernous malformation or surgery during at least 6 months of follow-up.
- Occurred in
- 6% medical management and surgery
- 6% medical management alone
- Dose
- 12-15Gy
- Given at least 3 months after last bleed
- Avoid DVA
- Outcome
- Morbidity
- Post-treatment haemorrhages
- Present in 7% patients after SRS
- Adverse radiation effect.
- 6.7% data from Sheffield
- Seizure free
- CCM patients with medical refractory epilepsy reported that about 40–50% of patients were seizure- free at follow-up
- epileptogenic cavernomata demonstrated 87% of patients were without seizures after surgery compared to 64% after SRS (Hsu et al., 2007)
Pregnancy and CCM
- Provide prepregnancy counselling regarding genetic risk in familial form
- If the patient has a seizure disorder,
- consider the AED with the lowest teratogenic potential
- Advise folate supplementation if the patient is taking seizure medication during pregnancy MRI without contrast, if needed, during pregnancy or postpartum while breastfeeding
- Vaginal delivery is an acceptable mode of delivery unless recent haemorrhage or neurologic deficits preclude such
