General
CBF changes parallel these metabolic changes (i.e. flow-metabolism coupling).
- The regulatory changes involved in flow-metabolism coupling have a short latency (about 1 s) and may be mediated by regional metabolic or neurogenic pathways.
- In health, flow and metabolism are closely matched, with remarkably little variation in the oxygen extraction fraction (OEF) across the brain despite wide regional variations in CBF and CMRO2.
Correlates to CBF
CBF (ml/100g/min) | Condition |
>60 (approx) | Hyperemia (CBF > tissue demand) |
45–60 | Normal brain at rest |
75–80 | Gray matter |
20–30 | White matter |
<20: Ischemia | ㅤ |
16–18 | EEG becomes flatline |
15 | Physiologic paralysis |
12 | Brainstem auditory evoked response (BAER) changes |
10 | Alterations in cell membrane transport (cell death; stroke) |
Cerebral Blood Flow and Ischaemic
CBF (ml/100g/min) | Cell State | Time to Infarction | Consequences |
50 (20-80) | Normal | – | Normal |
<23 | Oligemia | >6 h | EEG slowing |
10-17 | Penumbra | Several hours | Flatline EEG, absent evoked potentials |
<10 | Death | Several minutes | Membrane pump failure |
Cerebral blood flow (CBF) and oxygen utilisation
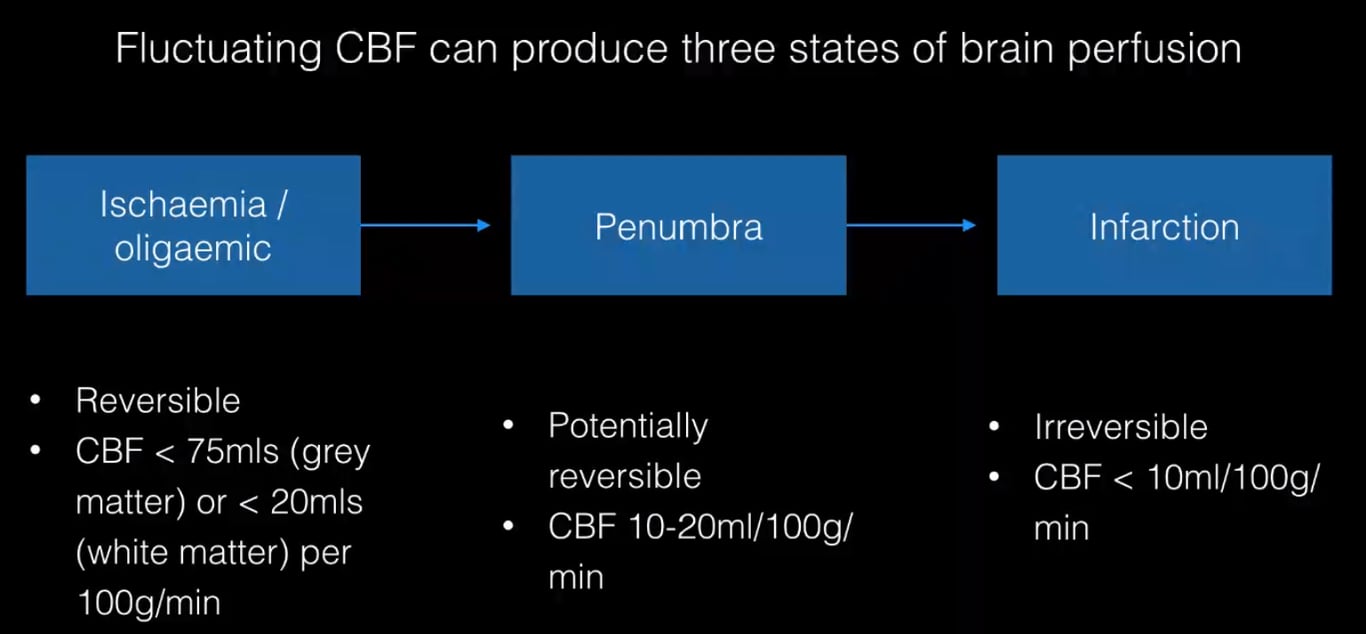
- CBF <20 is generally associated with ischemia and if prolonged will produce cell death.
- Assuming normal metabolic rate and may be more applicable to global cerebral hypo-perfusion.
- Penumbra
- Non functioning cells that are still viable.
- A region in which cerebral blood flow reduction has passed the threshold that leads to the failure of electrical but not membrane function. The neuron is functionally disturbed, but remains structurally intact
- Has a higher CBF threshold for loss of electrical excitability than that for cell death
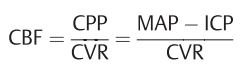
- CBF is related to blood pressure
- CPP= cerebral perfusion pressure
- CVR= cerebrovascular resistance
- MAP=mean arterial pressure.
Cerebrovascular resistance (CVR) and cerebral autoregulation
- CVR
- The resistance of the cerebral vascular bed to blood flow
- Affected by
- Changes in PaCO2
- Linear increase in CBF with increasing PaCO2 within the range of 20–80mm Hg.
- Changes in CPP
- Produces changes in blood vessel tone via a myogenic mechanism.
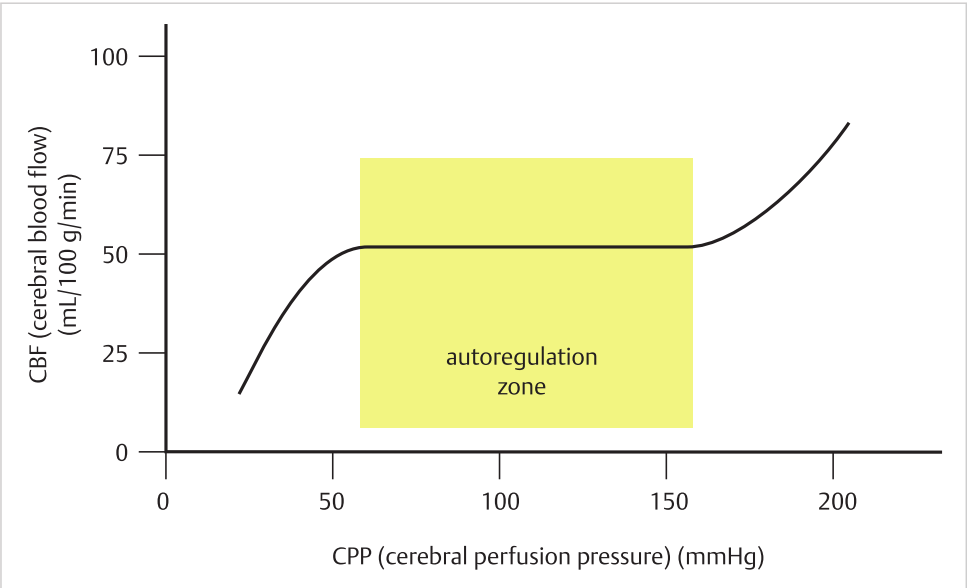
- Cerebral autoregulation
- Between CPP= 50–150mm Hg, the CVR of normal brain tissue varies linearly to maintain an almost constant CBF.
- Altered in pathologic states.
Cerebral metabolic rate of oxygen consumption (CMRO₂)
- CMRO₂
- Averages 3.0–3.8 ml/100 gm tissue/min
- The ratio of CBF to CMRO2 (the coupling ratio) in the quiescent brain is 14:18
- With focal cortical activity
- Local CBF increases ≈ 30%
- CMRO2 increases ≈ 5%
- CMRO2 can be manipulated to some degree
- Neuronal metabolism is heavily dependent on aerobic mitochondrial metabolism expending large amounts of energy for the ion pump systems to maintain membrane potentials and participate in action potentials.
- Substrates for neuronal aerobic metabolism are
- Glucose
- Lactate
- Ketone bodies
- End-products of neuronal activity are extruded into the extracellular environment.
- K+
- Glutamate
- Astrocytes act as metabolic sensors for the neuron (↑K+, ↑glutamate, ↓glucose, ↓lactate) which in turn is coupled to the microvascular endothelium allowing alteration of local blood flow related to local neuronal activity (regulation of cerebral blood flow (CBF)).
Cerebrovascular reserve and reactivity (CVR)
- Alterations in blood flow secondary to a vasodilatory stimulus (such as ACZ) can be used to estimate CVR, which is calculated as the percentage increase in CBF after ACZ relative to baseline
- Evaluated with
- Xenon-enhanced CT,
- CTP,
- TCD,
- SPECT, or
- MRI.
- Lower the reserve or reactivity high the risk of subsequent strokes
- Response of CBF to a vasodilator challenge (Stress) with 1000mg of IV acetazolamide (ACZ) (Diamox®)
- See Vagal 2009 et al
- Used in
- Strokes
- Moya-moya disease
- Pre- and Postoperative Evaluation of Extracranial-Intracranial Bypass for Flow Augmentation
- Carotid Balloon Occlusion
- Hyperperfusion Syndrome
- CO₂ reactivity post head injury
- Mechanism
- Carbonic anhydrase inhibitor → carbonic acidosis → induces a considerable increase in CBF.
- Systemic blood pressure, heart and respiratory rates, arterial pH, arterial CO2 pressure, and CMRO2 are unaffected.
- Protocol
- A standard dose of 1000 mg intravenously is used for the ACZ challenge test.
- Peak CBF augmentation occurs at approximately 10–15 minutes after intravenous bolus administration.
- A 30%–60% increase in CBF is achieved in healthy subjects
- Classified as
- Type I
- Normal baseline CBF with 30–60% increase following ACZ challenge
- Type II
- Decreased baseline CBF with blunted response of <10% increase OR
- < 10ml/100 g/min absolute increase after ACZ challenge
- Type III
- Decreased baseline CBF with paradoxical decrease of regional CBF following ACZ challenge,
- Suggesting a steal phenomenon in regions with maximally dilated vasculature at baseline
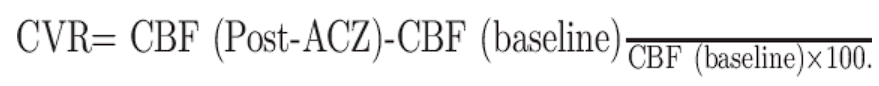
Secondary neuronal damage
- Produced by osmotically active serum proteins released by the clot
- BBB disruption
- Vasogenic oedema
- Failure of Na pump
- Cytotoxic oedema
Haemorrhagic transformation
- Due to reperfusion of ischaemically injured tissue due to
- Recanalization of occluded vessel
- Collateral blood supply to ischaemic brain
- Leads to disruption of BBB